Endorsement of Predicate In Vitro Diagnostics in Manufacturing License (Form MD 5) in India - MD-3 & MD-5

Are you a manufacturer of Class A or B notified medical devices in India, aiming for seamless market entry to distribute and sell your devices? CliniExperts is your one-stop solution for navigating the crucial Form MD-05 licensing process, efficiently and effectively.
Endorsement of Predicate In Vitro Diagnostics in Manufacturing License (Form MD 5) – Overview
Who Can Apply?
Application for endorsement is only available to manufacturers with the form MD 5 approval to manufacture the IVD.
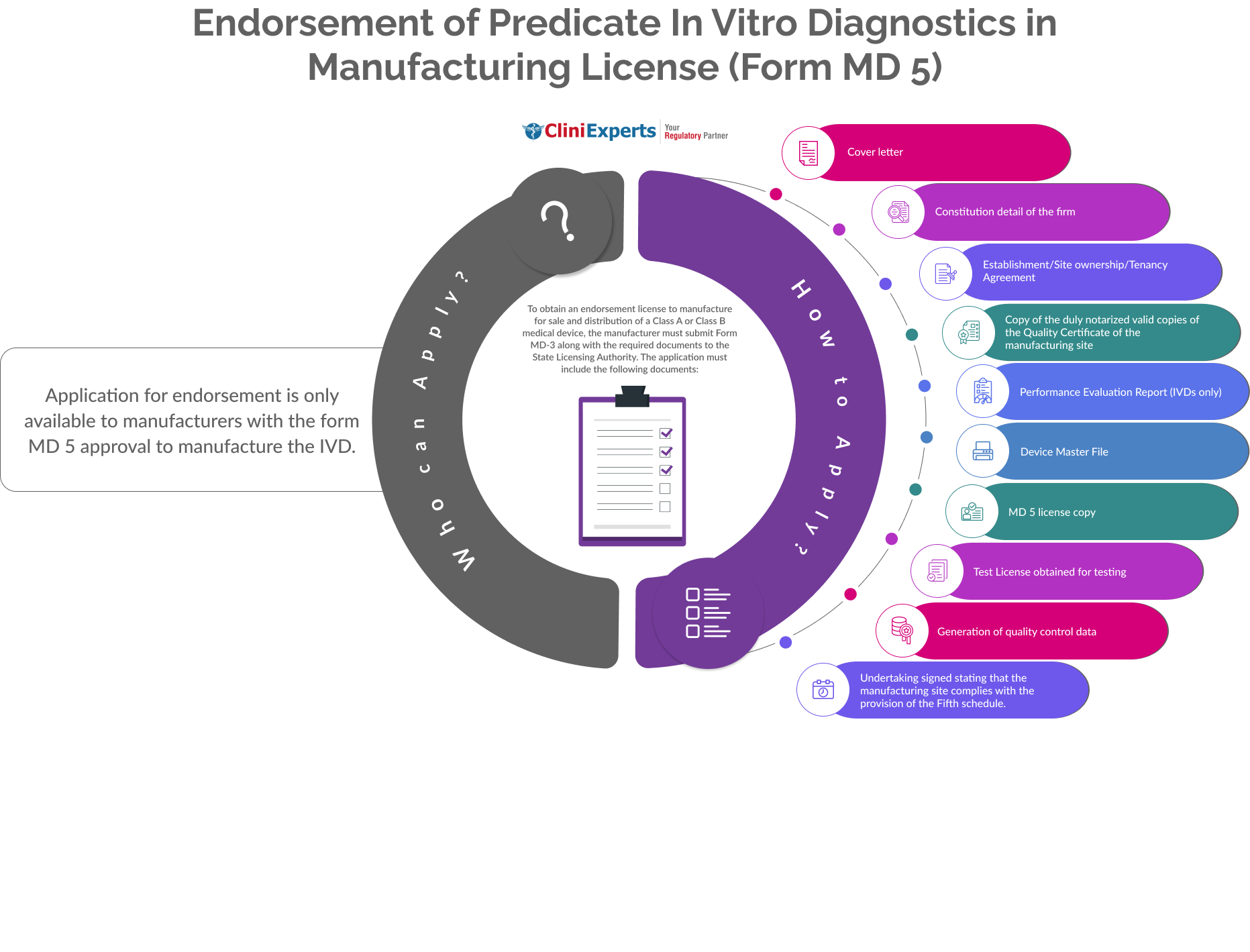
How To Apply?
The Applicant must follow the following process:
-

Cover letter
-

Constitution detail of the firm
-

Establishment/Site ownership/Tenancy Agreement
-

Copy of the duly notarized valid copies of the Quality Certificate of the manufacturing site
-

Performance Evaluation Report (IVDs only)
-

Device Master File
-

MD 5 license copy
-

Test License obtained for testing
-

Generation of quality control data
-

Undertaking signed stating that the manufacturing site complies with the provision of the Fifth schedule.

Validity
As long as the license is issued in Form MD-5, it remains valid for the duration of the license, provided that it is repaid within five years of its issue by paying the license retention fee as specified in the Second Schedule unless the license is revoked by the State Licensing Authority or suspended.
Fee Involved
There is a government fee of Rs. 500 for the application process of each distinct Medical Device of Class A or Class B.Important Documents

- Quality Management System (QMS) Documents
- Device Master File
Timeline to get
MD-5
from Central Drugs Standard Control Organisation
2-3
MonthsEssential Tips
Expert Advise
According to the experts, the manufacturers should generate quality control data based on a valid test license at the time of application submission.
Frequently Asked Questions
Is it possible for the manufacturer to select the notified body?
In accordance with the State Licencing Authority's advice, the notified body accredited under sub-rule (1) of Rule 13 of the Medical Device Rules, 2017 is authorised to conduct an audit of manufacturing sites for Class A and Class B medical devices to confirm compliance with the Quality Management System and other applicable standards as specified under these rules.

Does a manufacturing company still have to adhere to BIS standards if it is meeting ISO/IEC standards?

If devices that are currently on the market but have not been notified are added to the list of notified devices, would business continuity be taken into account?
The manufacturer of the device must abide by the Medical Device Rules, 2017 when it falls within one of the notified categories.

Would there be an exemption to the need for a local clinical investigation and evaluation for devices that are currently on the market and were announced later?
The medical device will be evaluated on a case-by-case basis and in light of available data to verify its efficacy and safety. The SEC may also be asked to hear the case.

