Wholesale Drug License in India - Form 20B 21B & Form 19

CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Wholesale Drug License (Form 20B, 21B) - Form 20B Drug License – Overview
Who Can Apply?
The applicant who wishes to store, distribute and sale the drugs in wholesale.
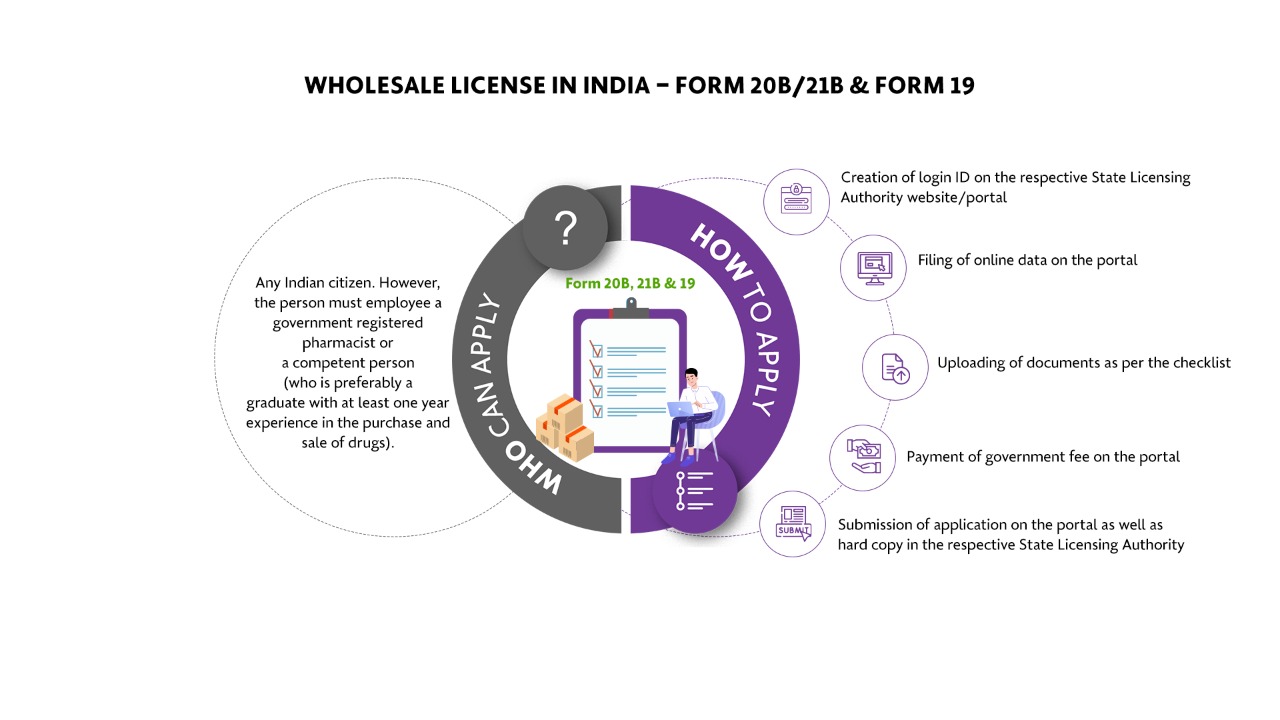
How To Apply?
The Applicant must follow the following process:
-

Creation of login ID on the respective State Licensing Authority website/portal
-

Filing of online data on the portal
-

Uploading of documents as per the checklist
-

Payment of government fee on the portal
-

Submission of application on the portal as well as hard copy in the respective State Licensing Authority

Validity
The wholesale drug license obtained would remain valid lifetime. Unless the regulatory authority suspends or cancels the license.

Fee Involved
For issuing a drug wholesale license, the applicant is required to pay 1500 INR for each license. The applicant must also submit a retention fee after every five years.Important Documents

To obtain a license for the sale or distribution of drugs in India, the applicant must submit the following list of documents:
- Layout and area of the facility
- List of directors, proof of ownership
- Proof of ownership, and partnership deed
- Biodata and credentials of the registered pharmacist or competent person
- Invoices for refrigerator and air-conditioning system
- System generated form and affidavits
Timeline to get
Form 19
from State Licensing Authority
21
WORKING DAYSEssential Tips
The applicant looking for permission license for the sale or distribution of drugs must ensure these essentials are followed
- The facility should meet the criteria of the State Master Development Plan, if any.
- The facility should not be less than 10 square meters.
- Proof of identities like aadhar card, pan card, and/or passport of directors and registered pharmacist.
- The registered pharmacist should have a graduate degree and one year of experience in dealing drugs.
- The owner should provide proof of ownership.
Expert Advise
The electric bill of the premises is a mandatory requirement.
If the commercial facility exists on a Delhi Development Authority allotted plot or flat, the Municipal Corporation of Delhi must issue a conversion charge receipt and necessary documents.
Related Services
Authorized Agent For DRUG in India
Importer | Regulatory Body: CDSCO
Understanding all the clinical regulations and complying with the standards to import drugs can be daunting. Experts at CliniExperts are here to help you at every stage of importing drugs, from undertaking to distributors (Form 9) to obtaining an import license (Form 10). We offer services to fill all required forms, complete documentation for an import license, pharmacovigilance, re-registration and post-registration regulatory support, all under one roof in a cost-effective manner.
Frequently Asked Questions
Can a wholesale license holder sell drugs in retail?
No, they can only sell drugs in wholesale.

Will the same wholesale license remain applicable during a change in constitution or shifting of premises?
No, the individual must apply for the respective change and get a new wholesale license.

Will an individual with no graduation degree but with more than five years of dealing with drugs be eligible to be a competent person?
Yes, but the person should be SSLC passed and have a minimum of four years experience in dealing with drugs.

Licensing Authority for Wholesale license?
State Licensing Authorities.

What is the validity of wholesale drug license?
The wholesale drug license is valid perpetually; however, retention fee shall be submitted every 5 years.

Is there common/single portal for applying drug wholesale license for each state?
No. Each and every state have their own respective portal. e.g.
- For Delhi: xlnIndia: https://xlnindia.gov.in/Home_xln.aspx
- For UP: niveshmitra: https://niveshmitra.up.nic.in/
- For Haryana: investharyana: https://investharyana.in/#/

Is single wholesale drug license used for Drugs, Biological and Medical Devices?
Yes. Wholesale drug license is same for medical device, drugs and Biologicals

