Import License for Medical Devices in India - Form MD 14 & MD 15

To get the Permission to Import Medical Devices, we meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Permission To Import Medical Devices Form (MD 14, 15) – Overview

Who Can Apply?
An authorized Indian agent must be appointed for the same. This agent must have a license to manufacture (for sale or distribution) or wholesale License FORM 20B & FORM 21 B (sale or distribution) as per the CDSCO guidelines. The agent will make an application to get the grant of medical devices import license by applying through the Sugam online portal.
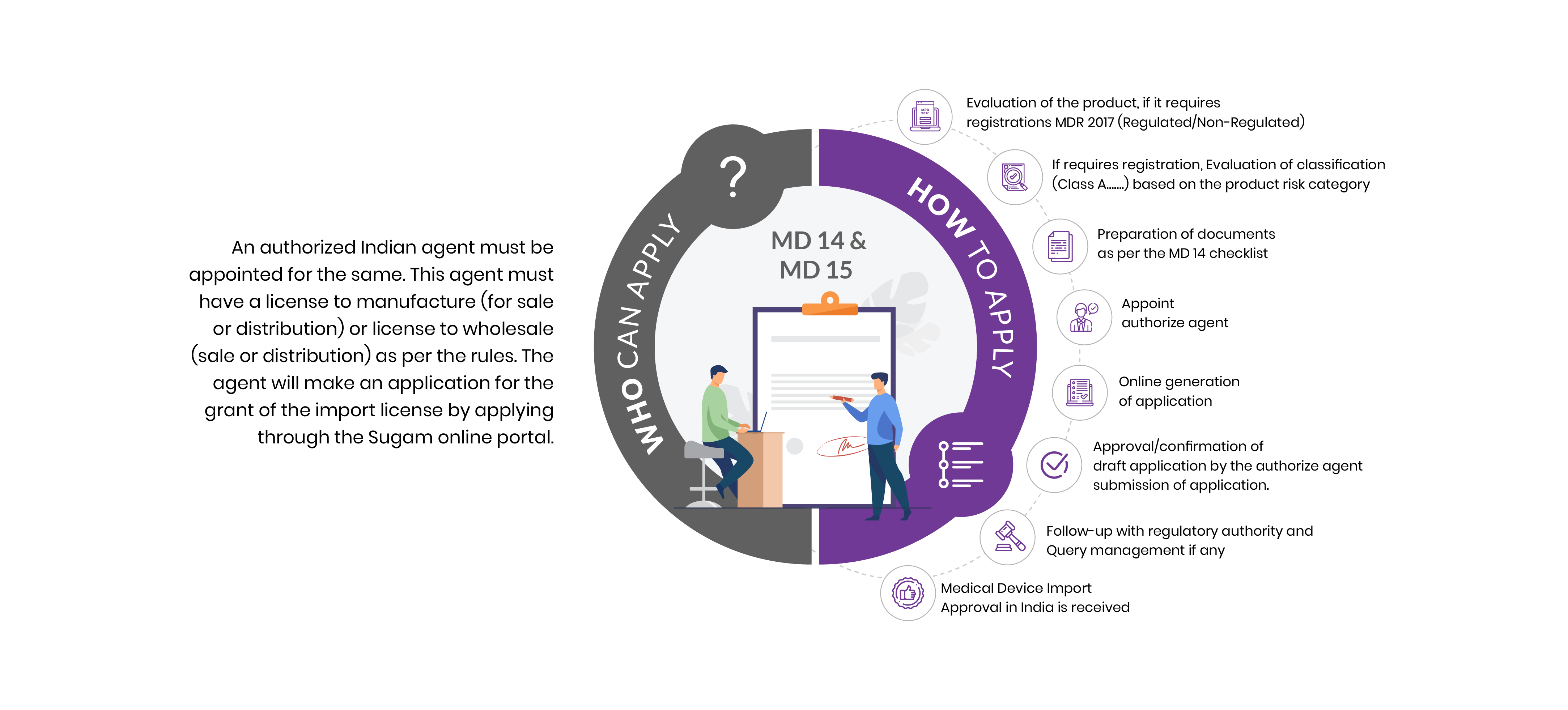
How To Apply?
The Applicant must follow the following process:
-

Evaluation of the product, if it requires registrations MDR 2017 (Regulated/Non-Regulated)
-

If requires registration, Evaluation of classification (Class A, B, C, & D) based on the product risk category
-

Preparation of documents as per the MD 14 checklist
-

Appoint authorize agent
-

Online generation of application
-

Approval/confirmation of draft application by the authorize agent submission of application.
-

Follow-up with regulatory authority and Query management if any
-

Medical Device Import Approval in India is received

Validity
The permission to import medical device is issued as Form MD 15 remains valid in perpetuity i.e., permanently, as long as the payment of license retention fee is done from time to time, as specified in the Second Schedule. The license retention should be paid each time before completion of the period of 5 years from the date of issue of the license unless it is suspended or cancelled by the Central Licensing Authority.

Fee Involved
Medical devices are categorised by risk class. Here is the correct fee structure for granting an import license- One site $1000: The fee to grant the permission to import medical devices for Class A medical devices other than in vitro diagnostic medical devices. Each distinct medical device cost - $50
- One site - $2000: The import license fee for Class B medical devices other than in vitro diagnostic medical devices. Each distinct medical device cost - $1000
- One site $3000: The import license fee for Class C or Class D medical devices other than in vitro diagnostic medical devices. Each distinct medical device cost - $1500
Important Documents

While making an online application for obtaining a Grant license for selling products, the applicant must upload the following documents:
- Wholesale License
- Power of Attorney
- Free Sale Certificate from GHTF
- Device Master File
- Site Master File
Timeline to get
MD 15
from CDSCO
6 to 9
MONTHSEssential Tips
Some Key points, you must pay attention before applying for the permission to medical devices import license:
- The Power of Attorney must be submitted in the same format as prescribed in the Medical Device Rules 2017.
- The Device Master Files, Site Master Files, and other technical documents need to be prepared as per the format prescribed in MDR 2017.
- In case there is no predicate device available in India, the applicant must obtain prior permission in Form MD 27 from the Central Licensing Authority. No license to an important class of such medical device shall be granted without such permission.
- Apostillation and notarization of the particular document must be followed as prescribed in MDR 2017.
Expert Advise
CliniExperts professionals strive to provide the unrivalled assistance to streamline the process of licensing and regulatory approvals to Import Predicate Medical Devices as per Medical Devices Rule 2017.
The Power of Attorney needs to be meticulously prepared as this document forms the basis of the entire application of the medical device Import License, India.
The product name and model numbers must be in alignment with the POA, FSC, Label and IFU, and other technical documents.
For Grouping of Products: It is crucial to apply the Grouping Guidelines on Medical Devices issued by the CDSCO. Failure to do the needful may attract additional government fees.
Related Services
Medical Device Authorized Agent - Registration Holder Support
Importer | Regulatory Body: CDSCO
CliniExperts can act as your authorized agent and provide end-to-end solutions to carry out the import of medical devices in India. The team of experts at CliniExperts facilitates the hassle-free one-time registration process and thus launch of medical devices in India in compliance with Indian regulatory requirements.
Clarification Letter / No Objection Certificate for Medical Devices in India
Importer | Regulatory Body: CDSCO
Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Test License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts' professional assistance
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Frequently Asked Questions
Can there be multiple importers for the same product?
Yes, there can be multiple importers for the same product, however all the applicants should meet the criteria for filling the application.

What are the documents required to get the import license for Medical Devices in case of multiple importers?
A new agent has to submit all necessary legal documents like MD 14, the new Power of Attorney, government fees, wholesale or manufacturing licenses, label, IFU, and a copy of the import license issued to the former agent. Additionally, an undertaking from the manufacturer must be submitted stating there is no change in the device master file, plant master file, and other regulatory documents submitted to CDSCO by the previous agent along with their name, address & import license number for registration. When in doubt, take the help of a consultant to make sure your documentation is in place.

What are the fees for manufacturing site if the importers wish to register devices belonging to multiple classes namely A, B, C, or D?
As per the Second Schedule, the manufacturer needs to submit the appropriate fees for different classes of the medical devices. If the organization is manufacturing all classes of the product, then the fees pertaining to the higher class need to be paid.

Will a change in the authorized Agent require a fresh license?
Yes, a change in the Indian authorized agent will require a fresh license application.

What is the retention fee for Class A, B, C, and D Medical devices?
The Import license retention fee for various medical devices is as follows:
One overseas site manufacturing Class A medical device other than in vitro diagnostic medical device - $1000; Each distinct medical device of Class A other than in vitro diagnostic medical device -$50.
One overseas site manufacturing Class B medical device other than in vitro diagnostic medical device - $2000 ; Each distinct medical device of Class B other than in vitro diagnostic medical device - $1000One overseas site manufacturing Class C or Class D medical device other than in vitro diagnostic medical device - $3000; Each distinct medical device of Class C or Class D other than in vitro diagnostic medical device - $1500

