Permission for Loan License to Manufacture Class A - B Medical Devices in India - Form MD 4 & MD 6

Achieve the success that your medical business deserves. Permission for medical device loan license to manufacture class A - B medical devices helps you to boost the production and attain new heights. CliniExperts guides you throughout the complete regulatory process to get the grant of loan license to manufacture medical devices in Form MD 6.
Permission for Loan License to Manufacture Class A - B Medical Devices – Overview
Any company which intends to obtain a loan license to manufacture Class A and/or B medical devices needs to make an application as per provisions of Medical Devices Rules, 2017. Medical devices classified as Class A and Class B are considered low and low-to-moderate risk devices, and they may also include in vitro diagnostic devices.
The manufacturer who wants to receive a grant for a Loan License for further sale and distribution of class A and B medical devices needs to file an online application through the online portal of the Ministry of Health and Family Welfare in the Central Government via the State licensing Authority. The application can be filled in Form MD-4 for Grant of Loan License to Manufacture for Sale or for Distribution of Class A or Class B medical device.
Who Can Apply?
Any person or company who wishes to manufacture Class A or Class B medical devices can apply for Form MD-4.
How To Apply?
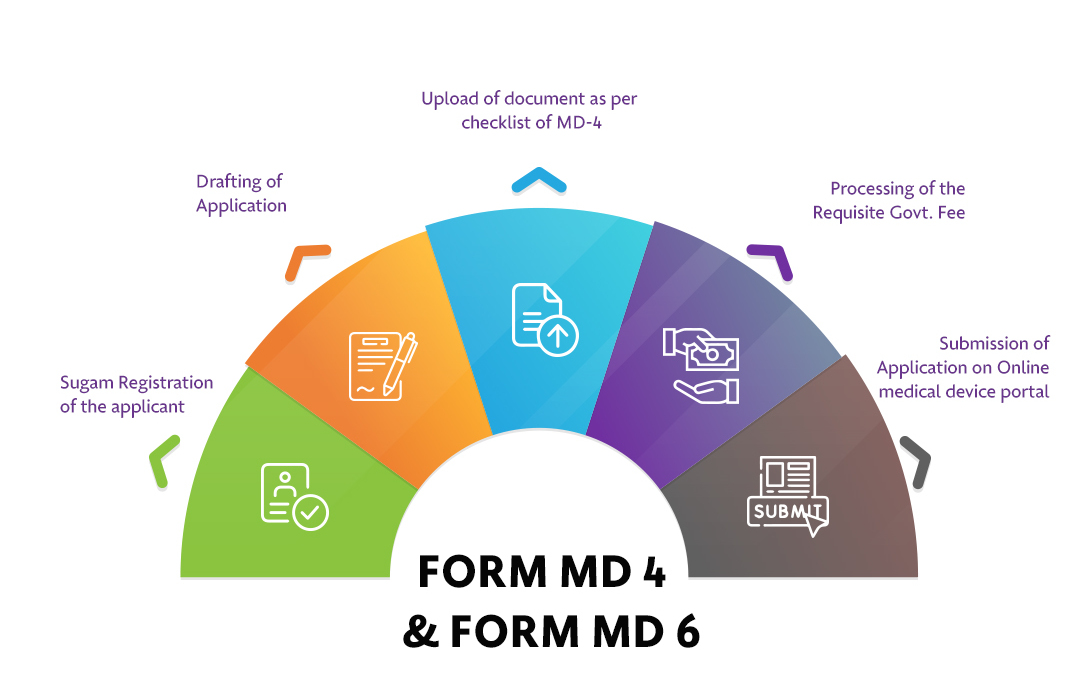
The Applicant must follow the following process:
-

Step 1: Sugam Registration of the applicant
-

Step 2: Drafting of Application
-

Step 3: Upload of document as per checklist of MD-4
-

Step 4: Processing of the Requisite Govt Fee
-

Step 5: Submission of Application on Online medical device portal

Validity
The validity of a license issued in Form MD-6 remains in perpetuity, provided that the license maintenance fee is paid as specified in the Second Schedule before the end of the period of five years from the date of issue, unless it is suspended or cancelled by State Licensing Authority.

Fee Involved
Loan License fee for single site manufacturing of class A / Class B devices - 5000 INRLoan License fee for each distinct Class A/ Class B medical device- 500 INR
Important Documents

- The Establishment/Site ownership/Tenancy Agreement
- Test License obtained from CDSCO
- QMS Certificate
- Plant Master File
- Device Master File
Timeline to get
MD 6
from State Licensing Authority
4 to 5
MONTHSEssential Tips
The essential processes which must be done for obtaining the grant letter for products coming under the Compulsory Registration Scheme (CRS) categories are:
- The essential tips to keep in mind during process of license application preparation and submission are:
1. Manufacturing site of the applicant must comply with the requirements of the Quality Management System as specified under the Fifth Schedule
2. Device Master files and site master files need to be prepared in accordance with the MDR 2017 format.
- The most possible roadblocks to consider are:
1. Technical documents of the medical device must comply with MDR 2017.
2. The primary manufacturer must have a license in accordance with MDR 2017.
Expert Advise
The quality control data must be generated on the basis of a valid test license when the application is submitted.
Related Services
SUGAM Registration- MD/ IVD in India
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch.
Test license to Manufacture Medical Devices in India – MD 12 & MD 13
Importer | Regulatory Body: CDSCO
CliniExperts dedicated team helps in getting the Permission For Test License To Manufacture Medical Devices. We strategically plan the process for regulatory approval of manufacturing test license for medical devices in Form MD 13.
Import License for Medical Devices in India – Form MD 14 & MD 15
Importer | Regulatory Body: CDSCO
Meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Frequently Asked Questions
What is the tentative time line for audit of the manufacturing site by Notified bodies?
The registered Notified Body shall carry out the audit of the manufacturing site within ninety days of the date of application in accordance with the Third Schedule.

In case an application is rejected, is there a provision to re-apply the license?
The aggrieved person may appeal to the State Government within 45 days of receiving such a rejection, and the matter may be disposed within 60 days after the investigation has been completed and the appellant is given an opportunity to be heard

Will the manufacturer have an option to choose the Notified body?
A Notified body accredited under sub-rule (1) of Rule 13 accredited shall be able to conduct an audit of the manufacturing sites of Class A and Class B medical devices to verify their compliance with the Quality Management System and the other applicable standards as specified by the State Licensing Authority for medical devices. Thus, manufacturers cannot choose the Notified Body.

