Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
July 14, 2016
File no. X11026/306/11-BD Dated 18 Jan 2013 In order to update the Quality information of already licensed products in a systemic approach it has been decided to submit the updated PPD of...

Blog
July 14, 2016
The "Guidelines on Similar Biologics" prepared by Central Drugs Standard Control Organization (CDSCO) and the Department of Biotechnology (DBT) lay down the regulatory pathway for a similar biologic claiming to be similar...

Blog
July 14, 2016
G.S.R. 588(E) – whereas certain draft rules further to amend the Drugs & cosmetics Rules, 1945, were published, as required by sections 12 and 33 of the drugs & cosmetics act, 1940...

Blog
July 14, 2016
The success of any new drug or FDC largely depends on the rationale and justification for using it. In line with the new regulations at CDSCO, all cases of new drugs, FDCs,...

Blog
July 13, 2016
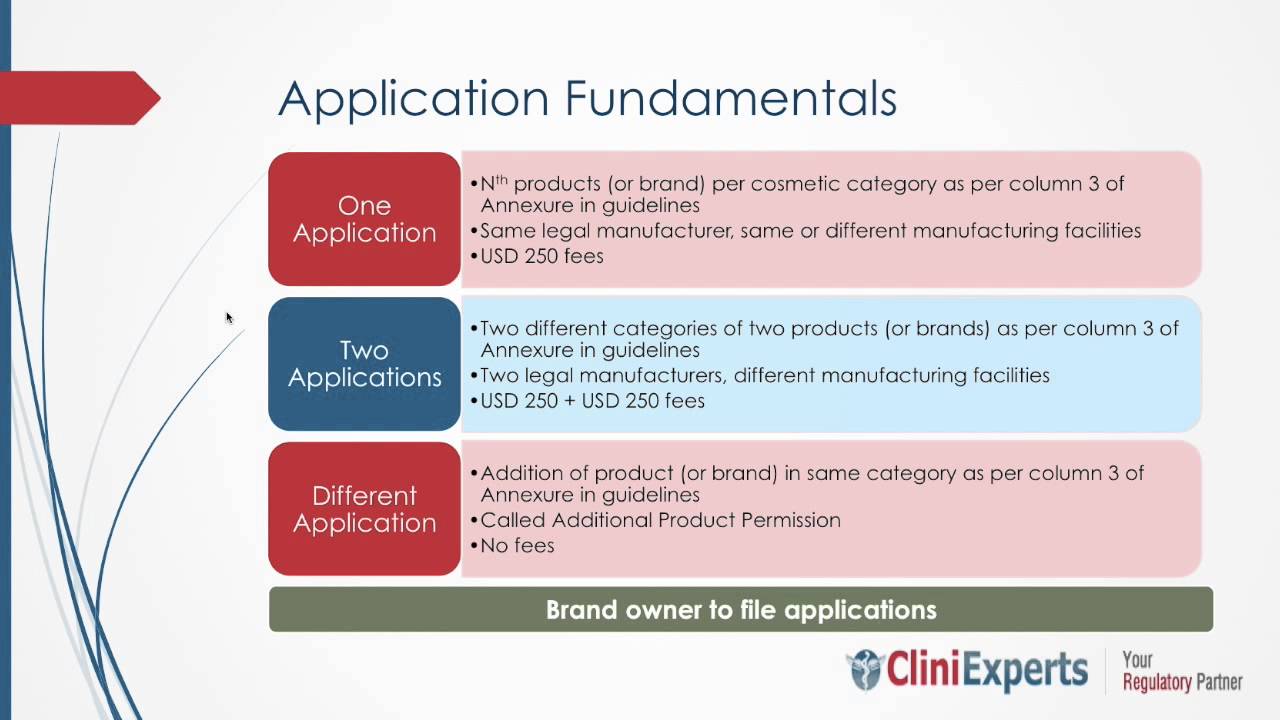
Cosmetic Regulations , Registration and Import Registration in India from CliniexpertsServices COSMETIC REGULATION - INDIA - Dr. Ashwini Kumar CEO CliniExperts Services (P) Ltd. akumar@cliniexperts.com Cosmetic- Definition Drugs & Cosmetic Act,...

Blog
July 13, 2016

Blog
July 12, 2016
As per the Drug and cosmetic act 1940, “cosmetic” means any article intended to be rubbed, poured, sprinkled or sprayed on, or introduced into, or otherwise applicated to, the human body or...

Blog
July 12, 2016
Physicians applaud the latest step in reducing new HIV cases, but insist that high-risk individuals who take the medication still need to practice safe sex. After decades of health professionals trying to...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding