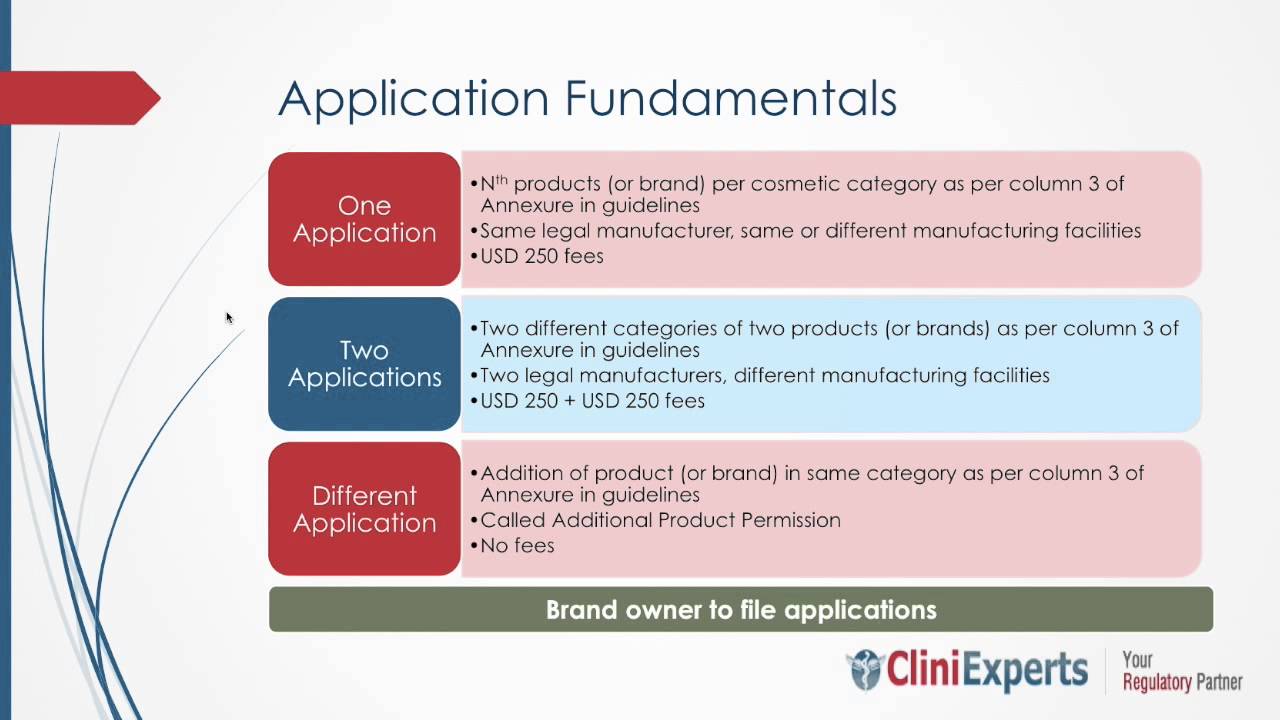
Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
July 10, 2014
As per the decision taken by the Expert Committee constituted by the Ministry of health & Family Welfare, all the Sponsors/ clinical trial applicants should ensure that the number of clinical trials...

Blog
July 9, 2014
NEW DELHI: Multinational pharmaceutical companies keen to conduct clinical trials for new drugs on Indians would have to ensure an early launch of those therapies in India if the trials result in...

Blog
July 5, 2014
It has been decided by the Expert Committee constituted by the Ministry of Health & family Welfare that all the sponsors/ manufacturers/ clinical trial applicants have to provide compensation to the trial...

Blog
July 1, 2014
By: David G. White, Ph.D. FDA Voice Today, “antibiotic resistance” is a widely recognized concern. With the rise of bacteria that are resistant to many, and in some cases, all...

Blog
June 29, 2014
FDA finalized three guidance documents that address various aspects of the use of nanotechnology in products regulated by the agency: Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology, Safety...

Blog
June 24, 2014
THE Union Health Ministry is working on a proposal to provide accreditation to clinical trial centres, investigators and ethics committees — all of which will kept be out of the purview of...

Blog
May 21, 2014
G.S.R 346 (E)- Whereas a draft of certain rules further to amend the Drugs and Cosmetics Rules, 1945 was published as required by section 12 read with section 33 of the Drugs...

Blog
April 25, 2014
Presently there is no specific provision under Drugs and Cosmetics Rules for payment of compensation in case of clinical trial related injury or death of the subject. However, the Good Clinical Practice...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding