Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
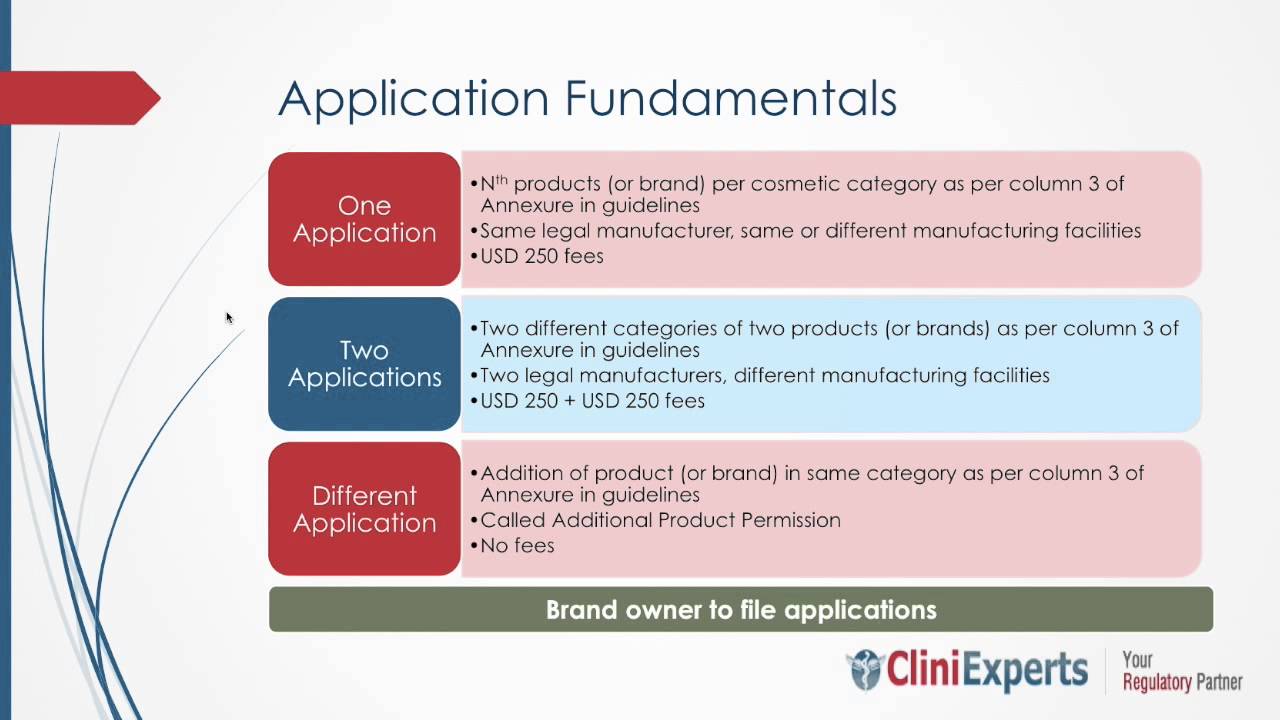
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Regulatory Update
November 14, 2024

Regulatory Update
November 14, 2024

Blog
November 4, 2024

Overview Navigating Software as a Medical Device regulation involves addressing varying global standards, including risk-based classifications. Challenges include managing risks, demonstrating clinical evidence, and ensuring data security. Harmonisation efforts and evolving frameworks...

Blog
November 4, 2024

Overview The future of (SaMD) Software as a Medical Device will see breakthroughs in Artificial Intelligence-based diagnostics, personalised treatments, and remote monitoring. Emerging trends emphasise real-time data integration, stronger regulatory measures, and...

Blog
November 4, 2024

Overview Software as a Medical Device refers to software applications designed for medical purposes, such as diagnosis, treatment, or monitoring, without being part of a physical device. Software as a Medical Device...

Blog
October 22, 2024

Overview Quality Management Systems for SaMD requires compliance with regulatory standards, implementation of robust quality management systems, and risk management through design controls, testing, and post-market surveillance. It also involves maintaining thorough...

Blog
October 22, 2024

Overview Clinical validation of Software as a Medical Device (SaMD) ensures a software's safety and effectiveness for its intended medical use. It involves rigorous testing and evidence collection to demonstrate that the...

Regulatory Update
October 16, 2024
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding