Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
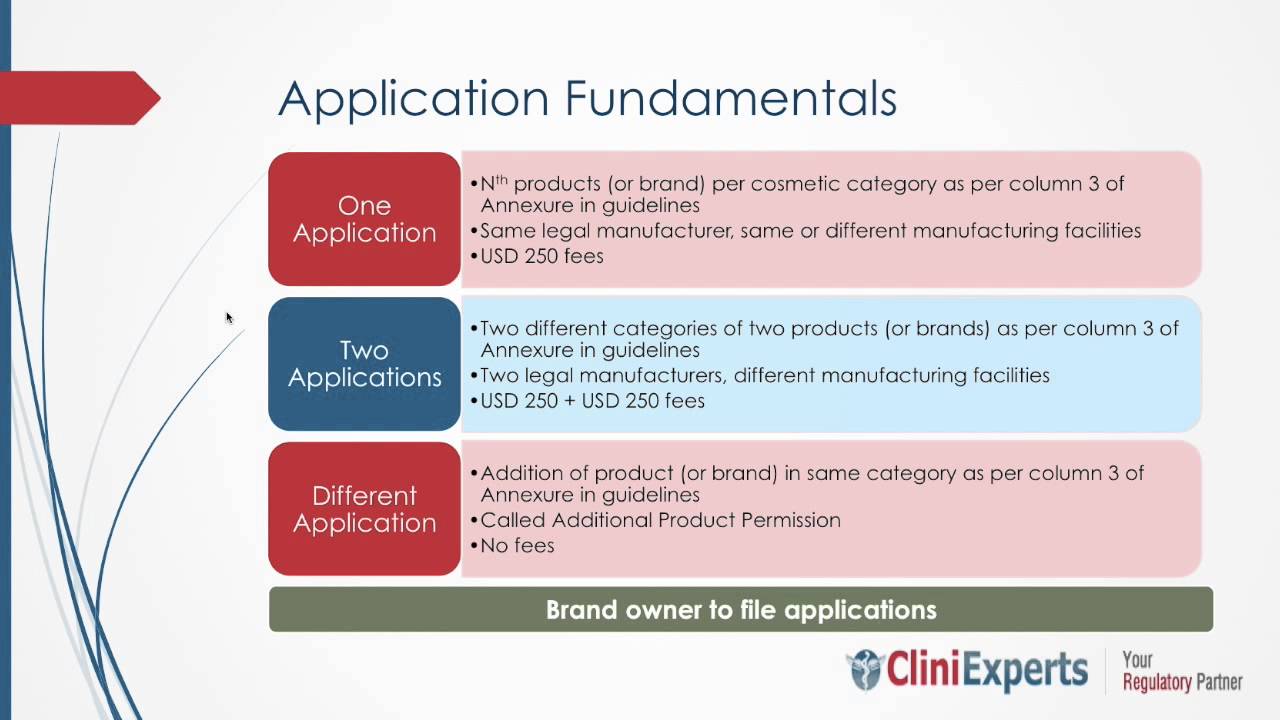
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Regulatory Update
September 11, 2024

Regulatory Update
September 4, 2024

Regulatory Update
September 4, 2024

Blog
August 13, 2024

All organizations engaged in the food industry in Delhi are required to obtain the Food Safety and Standards Authority of India (FSSAI) registration, commonly called the Food License. The Food Safety and...

Regulatory Update
August 10, 2024

Regulatory Update
August 1, 2024

Regulatory Update
July 3, 2024
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding