Table of Contents
What is the purpose of CDSCO SUGAM portal registration?

CDSCO is the National Regulatory Authority (NRA) of India . CDSCO launched the online e-Governance portal, SUGAM on the 14th of November 2015 for the purpose of licensing requirements of new drugs. The primary purpose of SUGAM portal is to provide transparency and accountability in all licensing procedures across India.
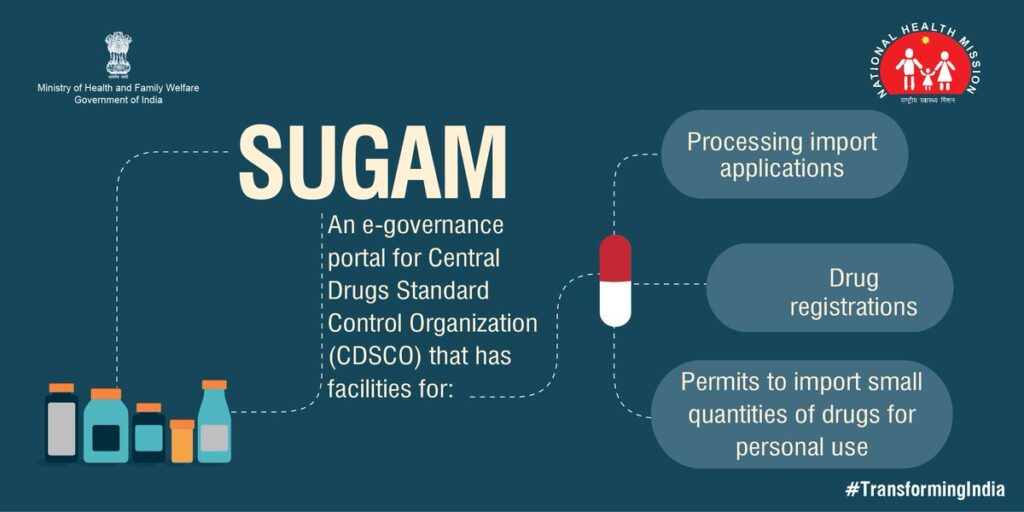
In a huge country like India, all types of drugs imported and manufactured in the country need to be stringently and heavily monitored. Hence, drug licensing and approval processes need to be put in place to help the Central and State government regulate the quality of drugs sold.
Due to this, the CDSCO launched SUGAM, an online e-Governance portal on the 14th of November 2015. CDSCO SUGAM portal is an online interface portal which helps businesses to easily access their licensing requirements and establish complete transparency throughout the entire process.
Central Drug Standard Control Organisation (CDSCO):
The import, manufacture, sales, and distribution of drugs is regulated by the Drugs and Cosmetics Act, 1940. Any substance defined as a drug and the manufacturer of drugs must also be registered with the CDSCO.
The statutory body responsible for regulations under this Act is the CDSCO. The purpose of CDSCO is to provide transparency, accountability and simplicity to all manufacturers and businesses for their drugs and Cosmetic licensing needs. This led to the launch of the online e-Governance portal, SUGAM.
What is CDSCO SUGAM Portal?
SUGAM is an online portal which was launched for grant of permissions and approvals related to drugs and import of cosmetics across India.
In order for manufacturers to obtain their license, they must first register on SUGAM with an application form along with the mandatory documents. Only after approval from CDSCO officials, can they login and access online SUGAM portal.
It consists of the following features:
- It consists of a vast database of all licenses for manufacturers, importers, businesses etc. granted by the State authorities.
- It has a wide variety of applications for different purposes such as NOCs, approvals, licenses etc.
- It also consists of educational information on various types of drug licensing procedures.
SUGAM has also made it very simple for its applicants to pre-register and register for their licensing. The steps are as follows:
- Pre-registration: The applicant must fill in the online registration form and submit it along with the hard copies of all mandatory documents (to the CDSCO office) such as ID proofs, address proofs, applicant undertaking etc.
- Registration: After close evaluation of the submitted application and documents by the CDSCO authorities, they will either approve it or reject it. Upon successful approval, the applicant shall receive their SUGAM portal login details by which they can now login and further register their business using the appropriate forms.
- Patek philippe replica: Timeless Classic
- The Calatrava collection by Patek Philippe embodies timeless class. These watches are known for their sleek, minimalist design, making them the perfect choice for any formal event. With iconic features like the hobnail pattern bezel and refined dials, Calatrava watches are a true symbol of meticulous craftsmanship.
Aim of SUGAM Registration:
The primary aim of the SUGAM online e-Governance portal is as follows:
- To establish a single interface for multiple stakeholders of a business/businesses to check their drug licensing processes.
- To establish transparency in all regulatory services of drug licensing.
- Enable paperless transactions for all CDSCO related procedures.
- Widen outreach of India’s standards and quality of drugs.
Purpose of SUGAM Portal Registration:
The primary purpose of the SUGAM online e-Governance portal is to provide a simple and practical interface for all the functions carried out by the CDSCO and for drug licensing, NOCs, permissions, and approvals’ needs of all manufacturers and businesses across India.
The secondary purposes of SUGAM are as follows:
- Tracking and management of online applications for drug licensing procedures.
- Establishing a simple platform for CDSCO officials to process E – applications, grant permissions and approvals, and generate MIS reports.
- Enabling improvements in facilities for the pharmaceutical industry and drug regulatory authorities in India. In contrast to traditional application processes, SUGAM has brought about clarity and reduction of delays in different types of licensing.
Conclusion:
In conclusion, SUGAM has brought about a huge improvement in the Indian pharmaceutical industry and a huge step in the digitization of Indian initiatives.
References:
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


