Table of Contents
Regulatory Challenges of SaMD: Navigating Global Standards and Compliance

Overview
Navigating Software as a Medical Device regulation involves addressing varying global standards, including risk-based classifications. Challenges include managing risks, demonstrating clinical evidence, and ensuring data security. Harmonisation efforts and evolving frameworks for SaMD aim to simplify compliance while accommodating innovation in digital health technologies.
Short Summary
- Software as a Medical Device refers to software intended for one or more medical purposes that functions independently of any hardware medical device.
- While Software as a Medical Device holds significant potential to enhance the healthcare system, it also brings new challenges for both regulators and the industry, including issues related to tracking, cybersecurity, and interoperability.
- The International Medical Device Regulators Forum is working with medical device stakeholders to achieve regulatory convergence and harmonisation in this field.
What Is SaMD?
The International Medical Device Regulators Forum (IMDRF) defines Software as a Medical Device (SaMD) as software designed for one or more medical uses, that accomplishes these functions independent of any accompanying hardware medical device.
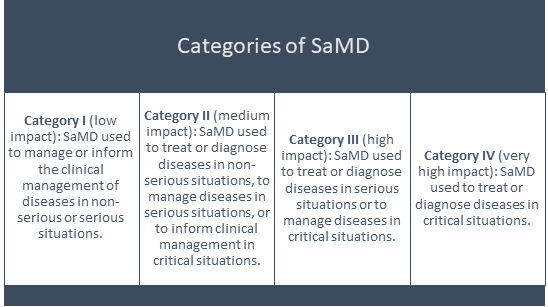
SaMD Categories
SaMD products can be categorised as follows:
Regulatory Challenges
Digitalisation is transforming healthcare, offering benefits like better access to healthcare, communication, and patient engagement. As SaMD usage grows, so do the regulatory challenges.
Regulatory frameworks for medical devices and treatments were originally designed for traditional methods like medications and physical devices and may not fully address the unique challenges and risks associated with SaMD.
Due to this, they can limit innovation in SaMDs, such as apps and Artificial Intelligence (AI) tools used in healthcare. There is a growing demand for expedited regulatory processes and incentives for streamlined patient care processes, virtual healthcare, and emerging technologies like machine learning and artificial intelligence.
This is especially relevant for SaMD, which often incorporates these advanced technologies to improve healthcare delivery.
As SaMD becomes more common, the main challenges that emerge include:
- Varying regulations for SaMD in different countries.
- The increased risks associated with these tools.
- The need for stricter regulatory oversight, which can impact development and investment.
The Food and Drug Administration (FDA) has set up the Digital Health Center of Excellence to address these issues and modernise the industry. While SaMD offers the advantage of rapid adaptation and advanced technology, it also presents challenges in maintaining patient safety and effectiveness.
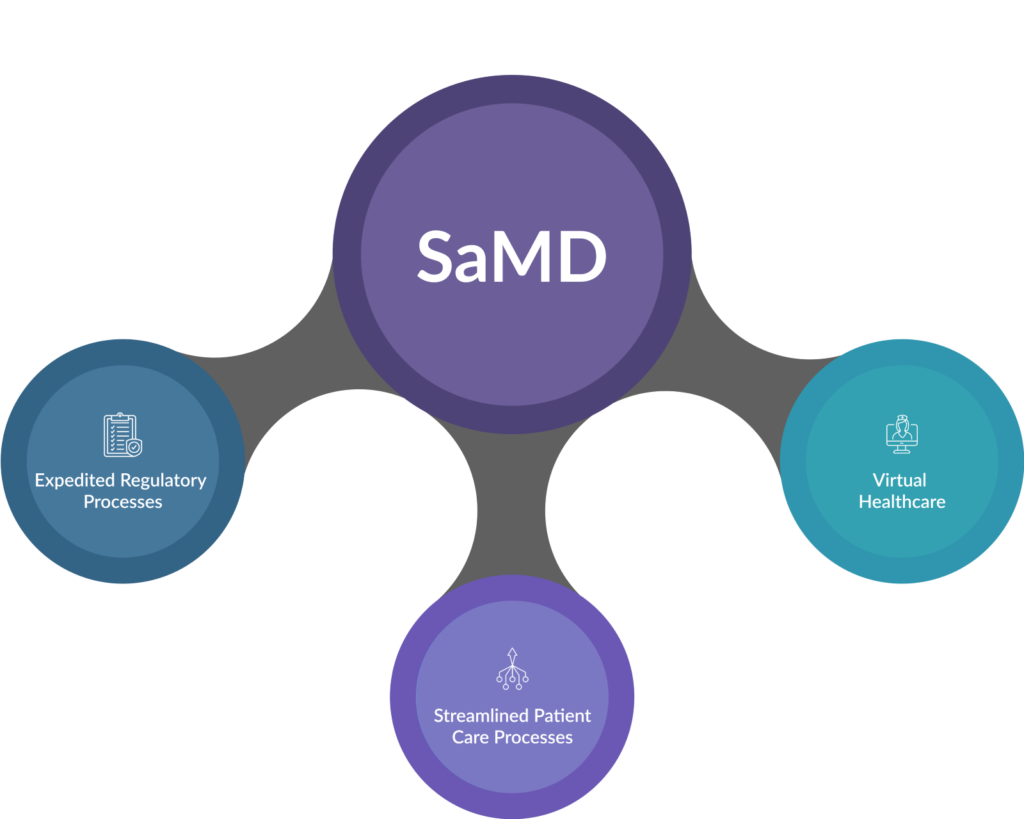
Fig. 1: Benefits of SaMD in Healthcare Delivery
Evolving Regulatory Landscape
The challenge with regulating SaMDs is that the rules and processes established at the start of development quickly become outdated as technology evolves. This continual outdating is a major issue for both the industry and regulators, who must ensure safety and performance.
The rapid development of diagnostic and therapeutic software is far outpacing the current regulatory systems, which are often too rigid and slow to keep up with the fast-evolving technology.
Addressing Regulatory Concerns
To overcome these challenges, certain steps can be taken, including:
- Lifecycle Monitoring: To enhance the quality and safety of SaMD, the product lifecycle should be optimized using post-market data to enable faster feedback and updates.
- Software verification and validation: Comprehensive testing should include real-world performance data to manage risks.
- Health Technology Assessment (HTA): HTA is essential for evaluating the safety, performance, and cost-effectiveness of medical devices. HTA can also influence reimbursement and funding decisions. To support regulatory requirements, HTA should consider innovative methods for evaluating SaMD and strive for consistent implementation across different regions to ensure reliable and uniform assessment processes.
- Regulatory Compliance: ISO 14971 and IEC 62366-1 standards aid regulatory compliance. In the EU, ISO 13485 is required, while in the US, FDA Quality System Regulation applies. Companies must also adhere to EU regulations on post-market surveillance and data protection (GDPR).
- Global Harmonisation: Global policies need to address safety and risk management in healthcare. This involves collaboration among regulatory authorities like IMDRF, EU, and USFDA to address the current fragmentation and ensure consistent safety and quality measures across countries. Currently, inconsistent rules and testing methods limit digital healthcare services. Global consistency is needed to improve healthcare access worldwide.
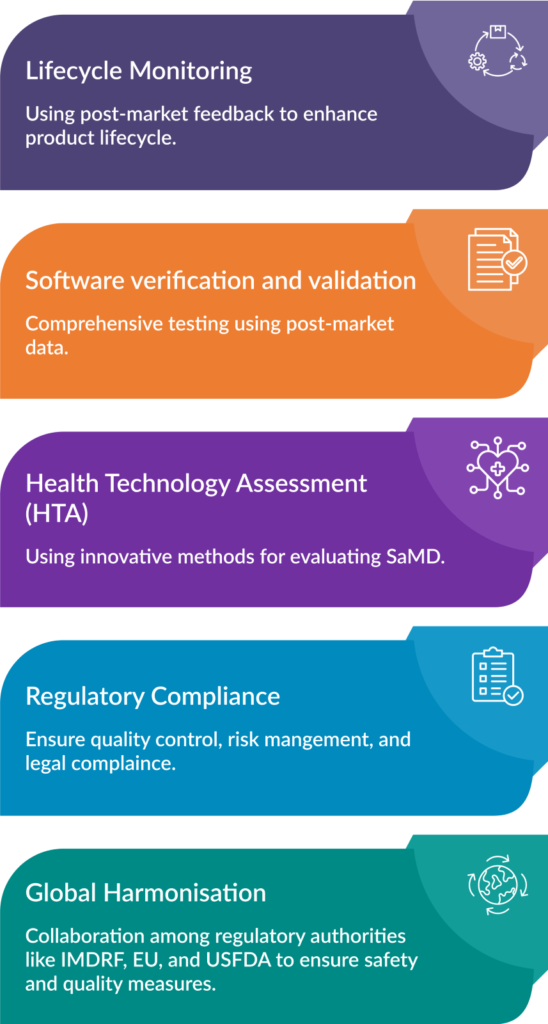
Fig. 2: Ways to Address Regulatory Roadblocks
Overall, a unified approach is essential to enhance global healthcare access through SaMD by ensuring consistent safety, risk assessment, and regulatory compliance.
Conclusion
Regulators can foster the development of SaMD by balancing strict safety standards with the need for innovation. This will ensure that SaMD technologies improve public health standards while keeping patient safety a top priority.
The goal is to establish a regulatory environment that ensures the safe, effective, and rapid deployment of SaMD. To achieve this, CliniExperts focuses on rigorous monitoring of emerging technologies, facilitating communication among stakeholders, and maintaining a commitment to adaptable, forward-thinking regulatory practices.
With these steps, SaMD technologies can reach their full potential and benefit patients and healthcare systems globally.
References
- Software as a Medical Device (SaMD) [Internet]. FDA. 2020.
Available from: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd
- Software as a Medical Device: Possible Framework for Risk Categorization and Corresponding Considerations | International Medical Device Regulators Forum [Internet]. www.imdrf.org. 2014.
Available from: https://www.imdrf.org/documents/software-medical-device-possible-framework-risk-categorization-and-corresponding-considerations
- View of Regulatory Challenges of SAMD [Internet]. Jchr.org. 2024.
Available from: https://www.jchr.org/index.php/JCHR/article/view/4857/3107
Recent Posts
Standards, Accreditation and Validity of FSSAI-Recognised Food Testing Laboratories

Short Description FSSAI strengthens food safety in India by accrediting laboratories through NABL, enforcing Good Laboratory Practices, addressing operational challenges, and promoting global st..
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


