Table of Contents
Safety and Efficacy Assessment in Non-Specified Food Approval: Focus on Functional Foods

Introduction
The Food Safety and Standards Authority of India (FSSAI) is a statutory body under the Food Safety and Standards Act, 2006 (FSSA, 2006) governed by the administration of the Ministry of Health and Family Welfare, Government of India. CliniExperts provide safety and efficacy assessment in non-specified food approval service in all over India. FSSA, 2006 is the primary law for the regulation of food products.
FSSAI has been created to lay down science-based standards for articles of food and to regulate their manufacture, storage, distribution, sale, and import to ensure the availability of safe and wholesome food for human consumption. Additionally, it guarantees that the FBOs adhere to the rules and dietary guidelines outlined in the FSS Act and regulations. It grants the FSSAI license which is necessary to operate a food business.
The Food Safety and Standards (Approval for Non-Specified Food and Food Ingredients) Regulations, 2017, initially established by the FSSAI, served as a framework for regulating non-specified food products and ingredients. However, recognizing the need for updates and improvements, the FSSAI amended these regulations on October 11, 2022.
The resulting Food Safety and Standards (Approval for Non-Specified Food and Food Ingredients) First Amendment Regulations, 2022, signify a significant step forward in refining and enhancing the regulatory landscape governing novel food items in India. These rules came into effect on the day they were published in the official gazette. A change has been made to Regulation 4 of the “Procedure for grant of prior approval.” The Food Authority must receive an application in FORM-I from FBOs of non-specified food, along with the necessary paperwork and fees. Based on the food articles’ safety evaluations, the application as per FORM-II may be approved or rejected.
This regulation covers the following foods and food ingredients:
- Novel food or novel food Ingredients processed with the use of novel technology
- New additives
- New processing aids including enzymes
- Non-specified food including new botanical, new fruit or fruit-based product, probiotic and prebiotic, or any other.
Functional foods play a crucial role in modern dietary practices, offering consumers the opportunity to incorporate health-promoting ingredients into their daily routines. However, the unique properties and potential health claims associated with functional foods necessitate a comprehensive assessment to ensure their safety and efficacy.
Under Annexure 1 FSSAI has described the methods to determine the safety and efficacy of the food materials:
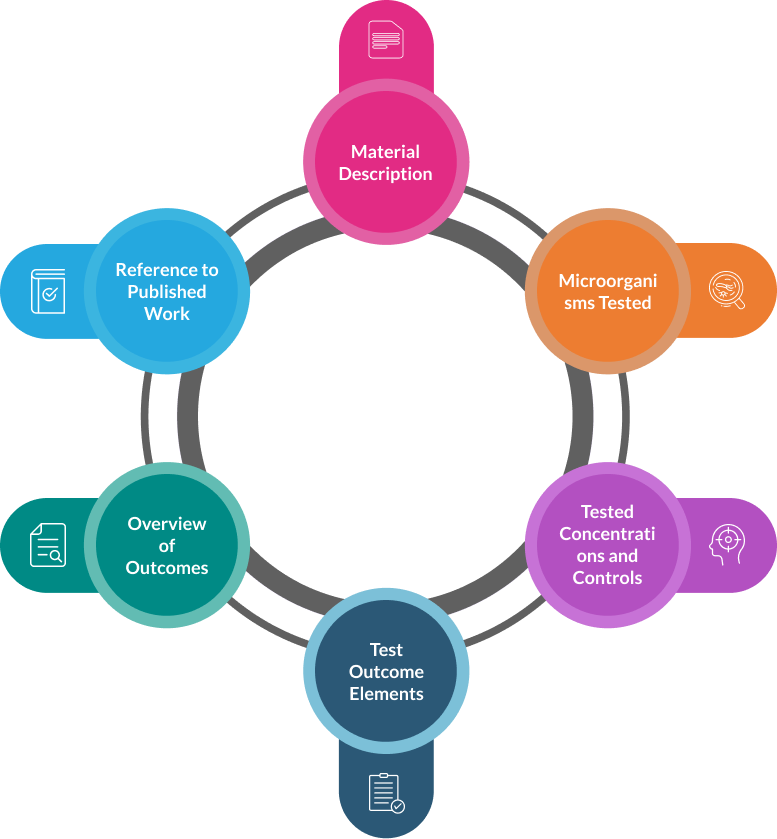
1. A description that offers an overview of the in vitro data
Describe the material that was tested, including its purity and, in the case of biological or botanical material, details on its standardization, marker compounds examined, and activity evaluated.
Provide details about the bacteria, yeast, or other microorganisms that were tested, such as their National Type Culture Collection Centre, Account Number, cell line details, organ or tissue culture details, or any other system.
Describe the concentrations that were tested as well as the positive and negative controls that were employed in the studies.
Describe the elements that the test measured as an outcome. For instance, cytotoxicity, IC50, dye uptake or decrease in dye uptake, and growth rate prevention.
Provide a succinct overview of the outcomes, a comparison to the controls, and any reported dose-response relations.
Give a thorough reference to the published work. Give specifics about the study, including the name of the laboratory or institute, its accreditation status, and where it was conducted. If the data is not published in peer-reviewed journals but is part of a study report, provide an authenticated copy of the report. Only provide a full paper copy of the most significant study and promise to provide copies of the other publications indicated in the table upon request.
2. Safety studies
Explain using test material that is suitable for regular human consumption.
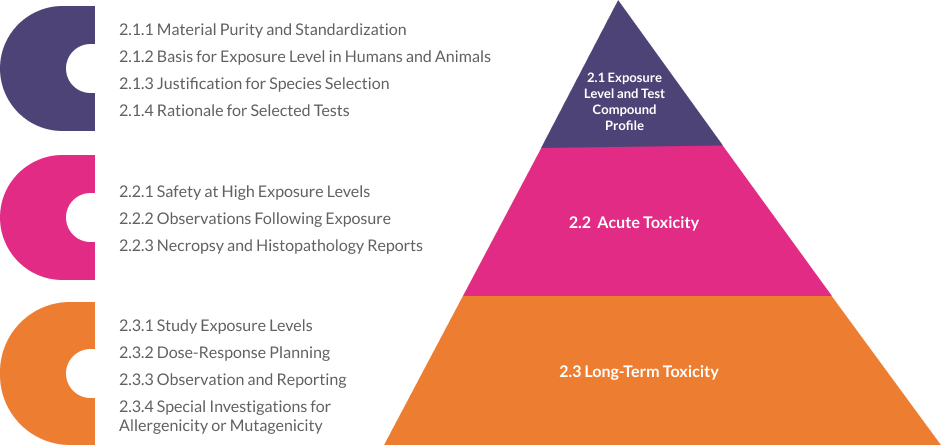
Exposure level and test compound profile
Explain the material that was tested for purity, including any standardization or marker compounds that were examined, in the case of botanicals, biological material, food, food additives, and color additives.
The intended /day/duration in humans should serve as the basis for the rationale when choosing the exposure level for experimental evaluation. The standard body surface area method should be used to determine the test exposure level for the chosen laboratory animal.
Give a justification for the species selection.
Explain the reasoning behind a few of the listed tests.
Acute toxicity
To ascertain the test substances’ toxic effects, ideally in two animals (mice, rats, or rabbits) exposed to the test compound in 24 hours.
A test compound’s safety at an exposure limit of 2 g/kg, or at least ten times the intended human exposure level and route, must be demonstrated in the study.
For 14 days following exposure to the test compound, the data should include observations on body weight gain, toxic signs, and the severity, onset, progression, and reversibility of the signs as well as any mortality.
The necropsy results and the histopathology report should be enclosed in the event of death.
Long-term toxicity
To ascertain the test substance’s toxic effects in two animals (mice and rats/rabbits) that are repeatedly exposed to it in accordance with its intended use for human consumption.
Three exposure levels must be used for the study: the intended human exposure level (Therapeutic Dose (TD)), High (5/10 X TD), Average (2.5/5 X TD), and The vehicle control group ought to be added as well.
To establish a no-observed adverse effect level (NOAEL) or other study objectives, dose level spacing should be planned to show a dose-response.
The information should include gross necropsy, histopathology, and physical, physiological, dietary, weight-gain, clinical chemistry, and hematological observations for the major and targeted organs. If an animal dies, a report might be included and the animals might undergo a gross necropsy and histopathology.
The reports on a special investigation must be included if the test material has the potential to be allergenic or mutagenic.
Conclusion
The safety and efficacy assessment of non-specified functional foods are critical steps in the regulatory approval process, ensuring that these products meet established safety standards and provide tangible health benefits to consumers. By adhering to rigorous assessment methodologies and regulatory guidelines, manufacturers can contribute to the development of a safe and innovative functional food market that promotes consumer health and well-being.
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


