Table of Contents
Understanding SaMD: What Makes Software a Medical Device?

Overview
Software as a Medical Device refers to software applications designed for medical purposes, such as diagnosis, treatment, or monitoring, without being part of a physical device. Software as a Medical Device helps healthcare professionals make informed decisions using data analysis and advanced algorithms and is regulated for safety and efficacy.
Short Summary
- Software has rapidly transformed nearly every industry over the past decade, including the healthcare sector.
- Software as a Medical Device is specifically developed to serve diagnostic, therapeutic, or monitoring functions in the medical context.
- Three key principles for Software as a Medical Device safety include risk management, quality management, and systematic and methodical systems engineering following industry best practices.
What Is Software as a Medical Device (SaMD)?
The International Medical Device Regulators Forum defines Software as a Medical Device (SaMD) as software applications designed for medical use that carry out functions normally associated with medical devices but do not require any physical hardware. These programs can be used independently to support, manage, or diagnose medical conditions.
Software is increasingly being used as a medical device. It can be utilized on a wide variety of technological platforms, such as virtual networks, “off-the-shelf” commercial platforms, and platforms for medical devices.
Categories of Software Related to Medical Devices
The categories of software associated with medical devices include:
- Software as a medical device: Examples include mobile apps that provide diagnostic information or software that helps manage chronic conditions. SaMD is regulated based on its intended use and the risks associated with it.
- Software as an accessory to medical device: Software utilized in the production or upkeep of a medical device, such as software that manages the data from a glucose monitor or provides updates and configurations to a medical device falls into this category.
- Software in a medical device: Software that is inherent to a medical device such as the software that controls the operation of an infusion pump or the firmware in a pacemaker. Without this software, the device cannot perform its intended function.
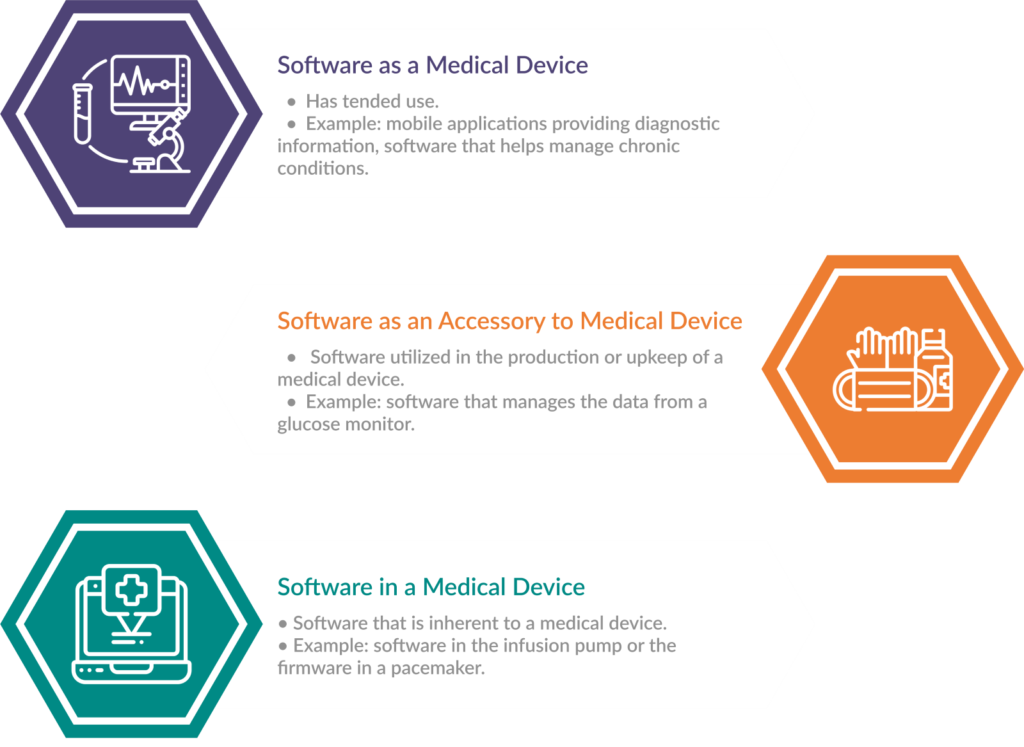
Fig. 1: Categories of SaMDs With Examples
Key features of SaMD
Essentially, SaMD is a software designed for medical use, whether standalone or integrated with other technology, with key features that include the following:
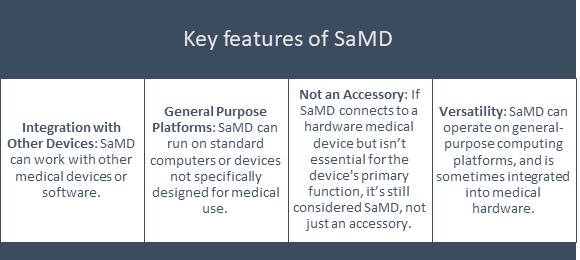
Examples of Software as a Medical Device
If the software is part of a hardware medical device, it does not meet the definition of SaMD. Examples of SaMD and those classified as not SaMD include:
| SaMD | Not SaMD |
| Treatment planning software, which provides data to a medical device like a linear accelerator. | Software used in an implanted pacemaker or other hardware medical device. |
| Apps that allow healthcare professionals to view MRI images on a smartphone for diagnostics. | Software that tracks how well a device is working to help with maintenance, such as checking x-ray tube performance to predict when it needs replacing. It also includes software that combines and examines laboratory quality control data to spot random errors or calibration trends in diagnostic equipment. |
| Computer-Aided Detection (CAD) software that helps spot breast cancer by analyzing images. | Software that uses data from a medical device but isn’t intended for medical purposes, such as software that encrypts data for secure transmission from a medical device. |
| Software for diagnosing a condition using a camera’s accelerometer. | Software designed to facilitate clinical communication and streamline workflow, including tasks such as patient registration, appointment scheduling, voice calls, and video calls. |
Safety of SaMD
SaMDs often integrate into clinical workflows to enhance diagnosis, treatment, and patient management. However, problems with the design or implementation of SaMD within these workflows can result in incorrect decisions by users and delays in decision-making, potentially leading to adverse patient outcomes.
Ensuring the safety of an SaMD involves identifying risks and implementing measures that confirm these risks are manageable. It is widely recognized that simply testing software is not enough to ensure its safe operation. Confidence in the software’s safety must be built through comprehensive strategies.
IEC 62304 is a standard that governs the life-cycle development of medical device software. It provides a risk-based decision model, outlines testing requirements, and focuses on the three key principles for SaMD safety:
- Risk management
- Quality management
- Systematic and methodical systems engineering following industry best practices

Fig. 2: Safety Plan for SaMDs
These principles collectively enable SaMD manufacturers to adhere to a structured and repeatable decision-making process that enhances safety.
Conclusion
Software is fast becoming a crucial component of many products with technological advances in healthcare. It is extensively integrated into digital platforms for both medical and non-medical uses.
Overall, SaMD represents a growing and dynamic field within healthcare, driven by technological advances and the increasing role of digital solutions in medical care; however, it also requires careful attention to regulatory, technical, and ethical considerations.
CliniExperts can provide the best expert advice and help you create high-quality SaMDs that can transform the quality of life for millions of patients. Contact CliniExperts and book an appointment today!
References
- Software as a Medical Device (SaMD) [Internet]. FDA. 2020.
Available from: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd
- What are examples of Software as a Medical Device? FDA [Internet]. 2022 Sep 27;
Available from: https://www.fda.gov/medical-devices/software-medical-device-samd/what-are-examples-software-medical-device
- Software as a Medical Device: Possible Framework for Risk Categorization and Corresponding Considerations | International Medical Device Regulators Forum [Internet]. www.imdrf.org. 2014.
Available from: https://www.imdrf.org/documents/software-medical-device-possible-framework-risk-categorization-and-corresponding-considerations
Recent Posts
Reduced Approval Timelines and Enhanced Compliance Obligations under NDCT Amendment Rules 2026

The Ministry of Health and Family Welfare, through G.S.R. 46(E) dated 20 January 2026, has implemented critical amendments to the New Drugs and Clinical Trials (NDCT) Rules, 2019. A major aspect of th..
Introduction of “Permission or Prior Intimation” Mechanism for Test Licenses under NDCT Rules, 2026

The NDCT Rules 2026 test license framework introduces a structured permission or prior intimation mechanism, streamlining regulatory oversight for investigational products, APIs, and clinical trial ma..
India-EU Free Trade Agreement (FTA): A Strategic Boost for India’s Pharma, Healthcare, and Food Sectors

The India-EU Free Trade Agreement (FTA) is a comprehensive trade framework aimed at liberalising bilateral trade and strengthening economic ties between India and the European Union. It enhances marke..
Need Help?
Submit your EnquiryOffice Locations
India
- Delhi
- Bangalore
Global
- USA
- Singapore
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


