What is Form MD 7 & MD 9, and How To Apply for Class C & D Medical Device Manufacturing Permission?

Medical devices in India are manufactured under the rules and regulations set by the regulatory authority of India, that is, CDSCO.
In India, to manufacture a medical device, one should have a manufacturing license from the CDSCO.
Class C – is a moderate-risk medical device, whereas; Class D – is a high-risk medical device.
Form MD 7: The application to grant a manufacturing license to sell and distribute class C and D medical devices.
Form 9: The permission of manufacturing license to sell and distribute class C and D medical devices.
| Application form | Approval form | Class | fees | Licensing authority |
| Form MD-7Application for manufacturing license | Form MD-9Permission for manufacturing license | C, D | Rs.50k for the manufacturing site;Rs.1k for per product registration | CDSCO |
1.1) Steps to get the manufacturing license in India:
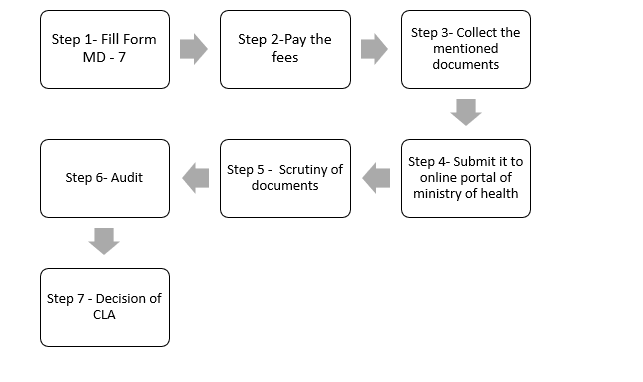
Step 1 – Fill out the Form:
- Fill the Form MD -7 at cdscomdonline.gov.in
Step 2 – Pay the fees.
- Applicants can pay fees under the head of the account through the government treasury challan.
- For the manufacturing plant, fees are 50k, whereas for the product is 1k per product.
Step 3 –Collect the documents:
- Technical documents as part II of the fourth schedule
- Cover letter
- Application form
- Fees challan receipt
- Firm constitution details – Partnership details, property declaration, list of partners along with age, and complete postal
- address
- Site ownership documents/ agreement of tenants
- Plant manufacturing details as per appendix I present under the III part of the fourth schedule, along with the declaration of manufacturing chemist, analyst chemist, educational qualification documents, and appointment letter,
- Each product’s device master file as per appendix II/III of the fourth schedule
- If applicable, a performance evaluation report
- Test license copy only if applicable
- Undertaking that site is compiled to the QMS as per the fifth schedule.
Step 4 – Submit all the documents to the online portal of the ministry of health and family welfare.
Step 5 – Scrutiny of application:
- CLA does it
- It takes approximately 40 days
- Any discrepancy is noted, and a query is raised
- The following action is taken upon the reply given by the applicant.
Step 6 – Audit
- The audit is conducted in manufacturing units by medical device officers and experts.
- It takes approx. 60 days to complete
- If any non – compliance is present can be rectified by the applicant.
- If the audit report is satisfactory, it will be sent for further action.
Step 7 – Decision by CLA
- If CLA is satisfied with the report, then a manufacturing license is granted in Form MD – 9.
- If CLA is not satisfied with the report, then; it rejects the application within 45 days with the rejection reason.
1.2) License Validity –
- The validity of Form MD – 9 is for five years.
- If, in any case, the license gets suspended or canceled, then the applicant can apply within 45 days from the action date.
1.3) Process of manufacturing license:
- Manufacturing license Form MD-7 is processed at zones and CDSCO headquarters
- A nodal officer can see the new application under the new application tab.
- The application is assigned to reviewing officer by the nodal officer who uploads the inspection report.
- If the previous reviewing officer is unavailable, then the Nodal officer can forward the application to another review officer.
- Under the new application file reviewing officer can view the file.
- The review officer generates the note sheet after they view/modify the checklist.
- The application is forwarded to the zonal DDA.
- Zonal DDA again reviews the file and generates the note sheet.
- If the inspection report is uploaded by the nodal officer of their corresponding zone, then the application is forwarded to the HQ DA.
- At DA of HQ, again file is reviewed, and the note sheet is generated and sent to LA/RO/SRO of HQ or NO/DDA of Zone.
- Again, LA reviews the file, generates the note sheet, and then approves the file.
- If the application is incomplete, then LA rejects the application and raises the query.
- At last, the application goes to the last assigned reviewing officer, who generates the permission, signs it digitally, uploads it, and shows the status as approved.
Summary –
- Previously, the manufacturers could sell their medical devices without any license, but now the government of India has made the manufacturing license compulsory.
- For classes C and D, the application for a license is audited by the CDSCO independently.
- As per Medical device rule (Amendment) rules 2020, it is mandatory to obtain a manufacturing license before Sep 2023 for classes C and D.
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


