Table of Contents
What penalties are levied in case of Non-Compliance in obtaining Wholesale Drugs?

Introduction
In accordance with the 1940 Drugs and Cosmetics Act, the license is granted by the relevant State Drug License Authority. The Drug and Cosmetics Act of 1940 combines all the drug-related laws. The drug must undergo a lengthy procedure before being used by patients, including numerous tests, cost-effective observations, and various stages, including laboratory, clinical tests, marketing, and sampling. In India, a drug license is required before opening a cosmetics and drug business. Manufacturers, retailers, wholesalers, and distributors who deal in pharmaceuticals or conduct business activities related to the pharmaceutical industry are granted drug licenses.
What is a manufacturing license – The license needed to start a business producing drugs, medicines, or cosmetics is known as a “manufacturing drug license,” and the appropriate authority grants it under the 1940 Drugs and Cosmetic Act. Under the 1940 Drugs and Cosmetics Act, the government has strict guidelines for approving the manufacturing and sale of drugs. The authorities also enforce the Food and Drugs Act and conduct routine inspections of the facilities and drug manufacturing facilities to prevent fraud. Ayurvedic, allopathic, homeopathic, and cosmetic medicines are all available.
The essential requirements for Obtaining a Wholesale Drug License
- The shop or office should be at least 15 square meters in size.
- The store or office needs a refrigerator and air conditioning.
- A few selected states will only grant a wholesale drug license to an organization or person with a degree or diploma from a reputable college or university.
- The wholesale drug license may be granted in the presence of a licensed chemist or other competent individual and under his supervision. Such a person ought to have either an SSLC with four years of experience in the drug-related field or one year of experience in the field with the latter.
- After obtaining the drug license, the owner must post it for future use outside the building housing his shop or office.
Advantages of Wholesale Drug License
The following list of advantages of having a wholesale drug license is provided:
Legality – A Manufacturing drug license application must be submitted because it gives the company legal status. Additionally, it is prohibited to manufacture or sell drugs in India without a valid license.
The authority can monitor the work – A wholesale drug license aids the authority in monitoring and controlling the distribution of medicines in India, ultimately improving the dependability and credibility of the business.
Business admissibility – A person must apply for a wholesale drug license because it gives the company legal standing. Additionally, it is prohibited to deal drugs in India without a license.
Evidence of authenticity – A manufacturing drug license is essential because it proves legitimacy and enhances the company’s reputation in the marketplace.
Increases the customer base – A drug license certificate demonstrates to your customers that your company adheres to stringent quality standards when producing drugs. Additionally, the license shows consumers that the medicines and drugs are safe and don’t contain any dangerous chemicals.
Prevent adulteration – The drug license guarantees that the products from the manufacturers are authentic in every way, so customers can use them without being concerned. It is one of the most sophisticated advantages of a drug license.
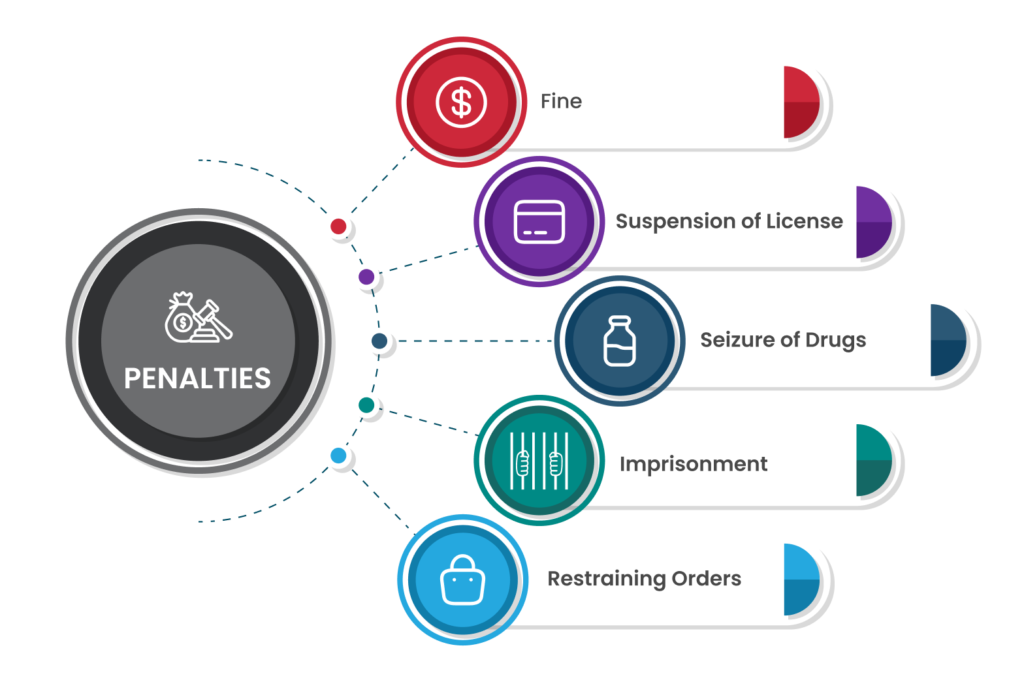
Penalties For Medicine Sales Without a Drug Licensee
- It can be extremely difficult and detrimental to the business to operate without a drug license. When rules are broken, people selling drugs without a license face punishment and imprisonment.
- If someone sells any medication without a drug license or without following the drug license process, as required by Section 18’s clause (c), they will be punished with the following:
- Imprisonment for a term that must not be less than one year and may not exceed three years, in which case the statute of limitations will apply in accordance with Section 468(2)(c) of the Criminal Procedure Code,
- Fine that must not be less than Rs. 5000
- The manufacturer, seller, or distributor in question will be sentenced to a term of imprisonment that shall not be less than five years, but which may extend to life if any drug manufactured or sold in accordance with these provisions is adulterated, spurious, or likely to cause any harm or death to the person in question. An alternative to or addition to imprisonment will be a fine of at least Rs. 10,000.
Conclusion
The penalties for non-compliance with drug licensing regulations can be severe. Obtaining a wholesale drug license is essential for legal compliance and demonstrates credibility and authenticity, leading to increased customer trust and business growth. Pharmaceutical business owners must abide by the regulations to ensure public safety and well-being and avoid severe legal consequences. Acquiring a wholesale drug license is a legal requirement in India for anyone engaged in the pharmaceutical industry. It ensures that businesses adhere to strict quality standards, maintain authenticity, and follow regulatory guidelines to protect the health of the population.
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


