
Blog
October 10, 2016
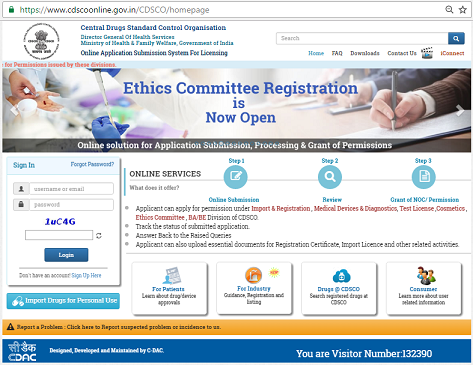
With rapidly growing digitalization in the world of healthcare and its accessory outfits, the Indian Government has chosen to join the foray and ride the digital wave. As part of implementation of...

Blog
September 7, 2016

India has been an attractive destination for clinical trials because of its large population and varied demographics. Despite having tremendous potential, only 1–2% of global clinical trials are being conducted in...

Blog
August 30, 2016
The Central Drugs Standard Control Organization (CDSCO) plays an important role in safeguarding and enhancing public health by ensuring the quality, safety, and efficacy of drugs, cosmetics, and medical devices. It is...

Blog
July 29, 2016
In order to strengthen the scientific review and approval of new drugs/devices, the ministry has appointed 12 New Drug Advisory Committee’s (NDAC), Subject Expert Committees (SEC) and 7 Medical Device Advisory Committee’s...

Blog
July 29, 2016
It is acknowledged within the pharmaceutical industry that clinical trials represent the single most expensive aspect of a drug, or treatment, development process. Specifically, clinical trials are enormously resource-intensive and involve repetitive,...

Blog
July 29, 2016
What is a New Drug as per Drug and Cosmetic Rules (D&C)? As per Rule 122 E of the Drug and Cosmetic Rules 1945, a New Drug can be – [1] A...

Blog
July 28, 2016
We are fully experienced, and have specialized skills in providing assistance to international pharma companies who want to register their products in the USA and the European Union. We are also...

Blog
July 28, 2016
Regulatory Affairs at US-FDA Drug & Medical Device Regulatory Consulting CliniExperts have aligned themselves with the internationally renowned regulatory consulting companies. These companies have a proven track record in drug, medical device, and regulatory consulting...






