In-Vitro Diagnostic Regulatory Services
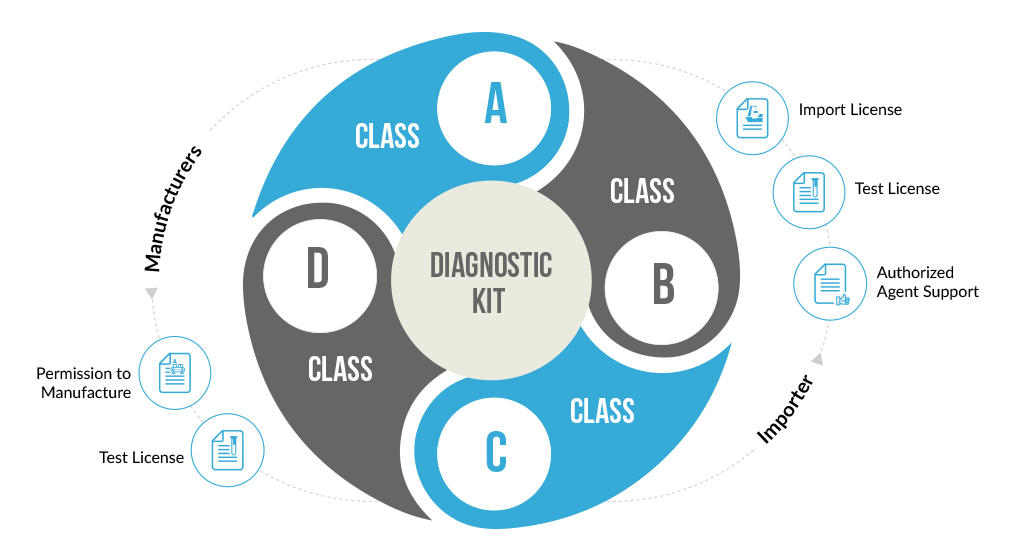
Diagnostic Kits play an important role in Medical science and are the base of almost every test, surgery or medical experiment. Pertaining to their importance and effectiveness in the medical field, the government of India has promulgated proper rules and guidelines for diagnostic kits in the New Medical Devices Rules, 2017, w.e.f 1st January, 2018. All the diagnostic kits whether used In-vitro or In-vivo are now regulated under the New Medical Device Rules, 2017. Diagnostic kits either manufactured in India or imported from foreign countries require to get the license for manufacturing, sale and use in the Indian market from Licensing Authority depending upon their classification.
Following the New Medical Device Rules, 2017, all in-vitro diagnostic devices and kits have been classified into four basic categories licenses for which are allotted by respective Central and State authorities. The categorization is based upon the complexity and risk involved with using the diagnostic kit.
New In-Vitro Diagnostic Device – First time in India
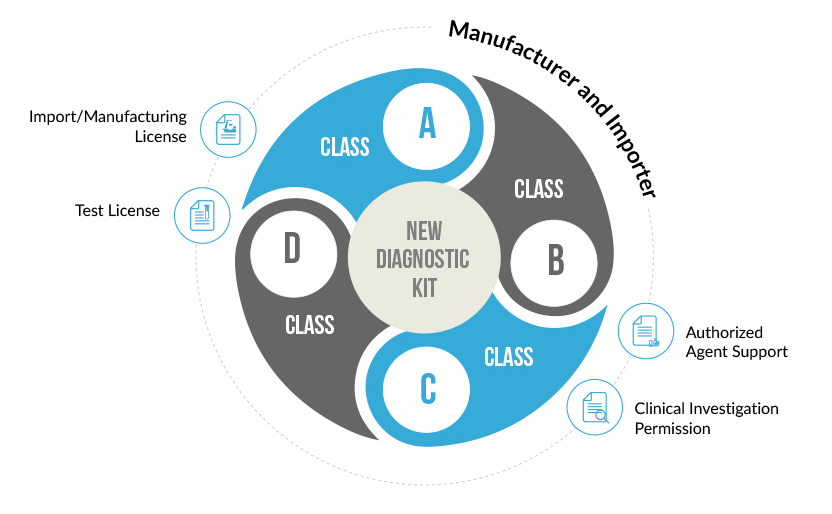
Any In-Vitro Diagnostic Device which does not have a predicate or similar device in the Indian market is considered to be a New In-Vitro Diagnostic Device. Such devices need to gain approval from the Central Licensing Authority for their manufacture or Import in India.
Classification of Diagnostic Kits in India
Based on New Medical Rules, the classification of the in-vitro diagnostic kits takes into consideration factors like the involved risk, medical condition being diagnosed, self-testing or near patient testing. IVD kits are used for serious medical conditions like HIV or Cancer are classified as high-risk devices and hence placed under Class D. Other simple kits like glucose testing strips, and sphygmomanometers are placed under Class A and B.
In-vitro Diagnostic Kits shall be classified in the following categories.
Risk Based Classification
Class A Low
Class B Low-Moderate
Class C Moderate-High
Class D High
The classification of the diagnostic kits takes into consideration factors like the involved risk, medical condition being diagnosed, self-testing or near patient testing. Diagnostic kits used for serious medical conditions like HIV or Cancer are classified as high-risk devices and hence placed under Class D. Other simple kits like glucose testing strips, and sphygmomanometers are placed under Class A and B.
Overview of forms for application
CDSCO has provided various forms to be used while filing the application for permission to import or manufacture diagnostic kits. The following table contains specific form which the applicant needs to be filled.
| Applicant | Risk/Class | Type of License | Forms |
|---|---|---|---|
| Importer | A, B, C, D | Importer License | Application: MD-14 Permission: MD-15 |
| Manufacturer | A, B | Manufacturing License | Application: MD-3 Permission: MD-5 |
| Loan License | Application: MD-4 Permission: MD-6 |
||
| C, D | Manufacturing License | Application: MD-7 Permission: MD-9 |
|
| Loan License | Application: MD-8 Permission: MD-10 |
||
| Importer | A, B, C, D | Clinical Performance Evaluation | Application: MD-24 Permission: MD-25 |
| Manufacturer | A, B, C, D | Clinical Performance Evaluation | Application: MD-24 Permission: MD-25 |
| Importer (New In-Vitro Device) | A, B, C, D | Import License | Application: MD-28 Permission: MD- 29 |
| Manufacturer (New In-Vitro Device) | A, B, C, D | Manufacturing License | Application: MD-28 Permission: MD- 29 |
For Importers
India has developed at a staggering rate as an economy. Hence the demand for medical care equipment has skyrocketed in the recent years. The new medical rules have classified all the existing diagnostic kits, including those which were earlier not classified. Filing an application for obtaining an import license is a simple single step process now. The process has been simplified to expedite the process and ensure proper availability of diagnostic kits in the country. The New Medical Device Rules allow multiple importers of a single diagnostic kit which was forbidden earlier. Although, each importer has to file a separate application for each diagnostic kit being imported.
Import License Process

Classification
of Medical Devices

Authorized Agent /
Registration Holder
Support

Aplication Filing
(Form MD- 14)

Import Licence
(Form MD- 15)
- Authorized Agent /
Registration Holder - Permission for Import License
(Form MD-14, Form MD-15) - Permission for test license to
import Diagnostic Kit
(Form MD-16, Form MD-17)
Authorized Agent / Registration Holder
All the applications for import of diagnostic kits will be filed by Authorized agent only. Foreign manufacturer cannot file an application directly to CDSCO. Authorized agent means a person including any firm or organization who has been appointed by an overseas manufacturer through a power of attorney to undertake import of diagnostic kits in India.
We at CliniExperts hold a valid Wholesale License (Form 20B and 21B) enabling us to act as Authorized agent for our clients which helps reduce the time of foreign manufacturer to set up their office in India and expedite the launching of the product.
Permission for Import License (Form MD-14, Form MD-15)
An application for issue of an Import License (Form MD- 15) shall be made to the central licensing authority in Form MD- 14. The authorized agent in India having a valid whole sale license for sale or distribution can apply for the Import License in MD-14 on behalf of the manufacturer. Our technical team at CliniExperts help our clients to obtain Import License.
Read More
Permission for Test License to Import In-Vitro Diagnostic Kit (Form MD-16, Form MD-17)
Any diagnostic kit can be imported in small quantities for the purpose of clinical investigations or test or evaluation or demonstration or training.
Importer who desires to import diagnostic kit, shall apply for an import license for test, evaluation or demonstration or training to the Central Licensing Authority in Form MD-16. Permission to import will be granted in MD- 17.
CDSCO has specified few diagnostic kits whose Performance Evaluation from NIB, Noida is mandatory prior to grant of license. For NIB testing, test license is required to be obtained. CliniExperts helps the client to obtain a Test License for testing and analysis of samples at NIB, Noida.
We at CliniExperts possess knowledge of the laws and regulations relating to the development, testing, approval and manufacture of kits.
For Manufacturer
New Medical Device Rules, 2017 have defined distinct provisions to obtain permission for manufacturing of diagnostic kits in India. Different diagnostic kits have been classified under different categories based upon their use, complexity and the risk involved. Applications for Class A and Class B diagnostic kits are reviewed and granted permission by the State Licensing Authorities. Whereas applications for Class C and Class D medical devices are reviewed and granted license by the Central Licensing Authority. This difference is obviously due to the depth and scale of review involved for different classes of diagnostic kits. CDSCO has also defined different fees for different classes of diagnostic kits.
Manufacturing License for Invitro Diagnostic Kits from SLA/CLAA
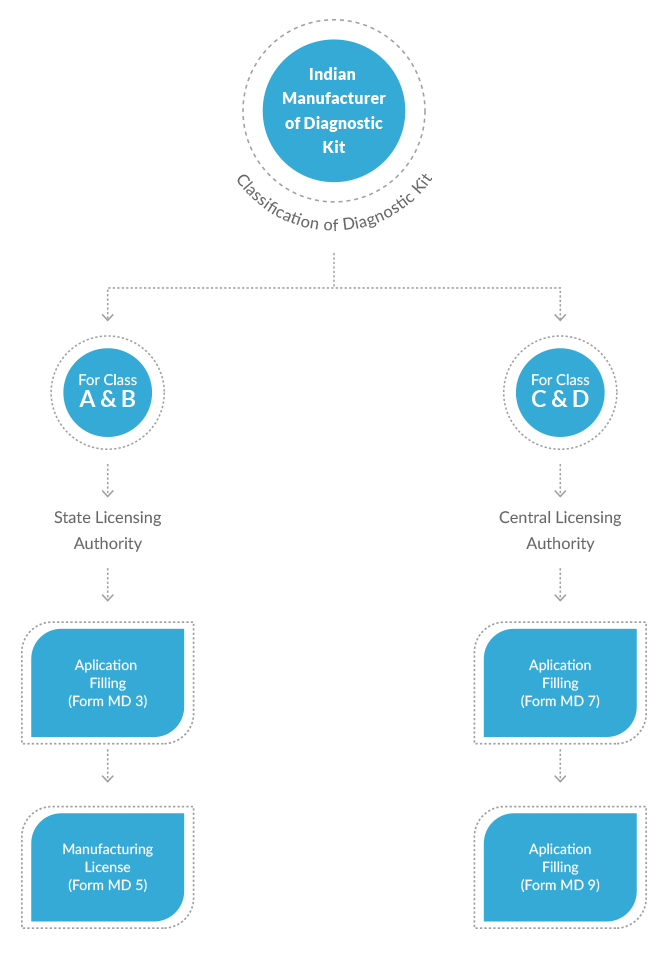
- Permission to Manufacture or Permission for loan license to manufacture Class A & B Diagnostic Kits in India from State FDA (Form MD-5 and Form MD-6)
- Permission to Manufacture or Permission for loan license to manufacture Class C & D Diagnostic Kits in India from CDSCO (Form MD-9, Form MD-10)
- Permission for test license to manufacture Diagnostic Kits (Form MD-12, Form MD-13)
Permission to Manufacture or Permission for loan license to manufacture Class A & B Diagnostic Kits in India from State FDA (Form MD-5 and Form MD-6)
Any company planning to manufacture Class A and B diagnostic devices must obtain a permission to manufacture from the State FDA. Since Class A and B devices as classified as low to moderate risk, and the application review process for them is quite simple and less stringent. Any manufacturer who needs a loan to manufacture diagnostic kits must first obtain a loan license from the State Licensing Authority to apply for a loan. Cliniexperts will help you in filing a manufacturing and/or loan license for Class A and B diagnostic kits
Read More
Permission to Manufacture or Permission for loan license to manufacture Class C & D Diagnostic Kits in India from CDSCO (Form MD-9, Form MD-10)
Class C and D diagnostic kits have a fair amount of risk involved with them, thus their license application and approval process is quite stringent. The permission to manufacture these devices is thus filed and obtained from the Central Licensing Authority using form MD-9. A manufacturer trying to procure a loan for manufacturing these diagnostic kits must obtain a license to apply for the loan.
Cliniexperts has helped a lot of clients file and obtain manufacturing and loan license for Class C and D medical devices. Our fantastic team of experts and regular follow-ups helps in expediting the process and improving the chances of approval.
Read More
Permission for test license to manufacture Diagnostic Kits (Form MD-12, Form MD-13)
Diagnostic kits can be manufactured in small quantities for the purpose of test, clinical investigation, demonstration or training. A test license to manufacture such diagnostic kits has to be obtained from the Central Licensing Authority. The test license can be filed for any class of medical devices. The application is filed using form MD-12 and the permission is granted in Form MD-13. Cliniexperts helps you in filing the application accurately to improve your chances of approval. Our team of experts ensures that the application process is hassle free and quick.
Read More
New In-Vitro Diagnostic
First Time in India
New In- Vitro Diagnostic Device are the devices that has not been approved for manufacturing or importing by Central Licensing authority
A New In-Vitro diagnostic kit is one whose similar or predicate device is not available in India. Such diagnostic kits need to undergo clinical investigations to prove their safety and effectiveness. This Clinical Performance Evaluation is conducted on specimens collected on voluntary human participants. Once the clinical performance evaluation has been completed a report describing the results of the investigation is generate. An application for the import or manufacture new In-Vitro Device is filed at the Central Licensing Authority, along with this performance evaluation report. After proper evaluation of the report’s findings, a permission is granted by the CLA to import or manufacture the diagnostic kit in India.
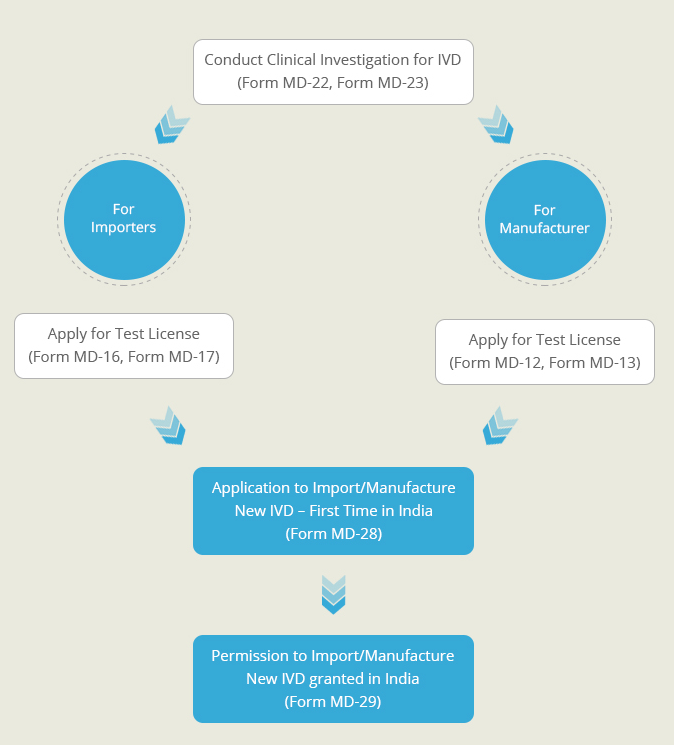
- Permission to conduct Clinical Performance Evaluation For New In-Vitro Diagnostic (Form MD-25)
- Permission to import/manufacture New In-Vitro Diagnostic which does not have a predicate kit (Form MD-29)
Permission to conduct Clinical Performance Evaluation for New In-Vitro Diagnostic (Form MD-25)
Any new in-vitro diagnostic kit has to undergo a clinical performance evaluation. The application to obtain permission for this evaluation for any class of medical device has to be filed at the Central Licensing Authority using Form MD-25. Preparing and filing the application in the right format is mandatory to ensure positive chances of approval. Cliniexperts has precisely trained staff to help you prepare the application and attach finding of the Clinical Performance Report in the proper format. Our skilled team and resources ensure that your application gets approved as soon as possible.
Read More
Permission to import/manufacture New In-Vitro Diagnostic which does not have a predicate kit (Form MD-29)
Once Clinical Performance Evaluation is done, the applicant needs to submit application for import/manufacture New In-Vitro Diagnostic. The application consists of data generated by the client.
Our team focuses on quality attributes as well the scientific rationale of the New device application.
Read More
Related Services
Wholesale Drug License in India
Importer | Regulatory Body: SLA
CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Clarification Letter / No Objection Certificate for Medical Devices in India
Importer | Regulatory Body: CDSCO
Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Test License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts professional assistance
Authorized Agent For In-Vitro Diagnostic Kits
Importer | Regulatory Body: CDCSO
Importing an in-vitro diagnostic kit and selling it across the country can be overwhelming if you do not have any local establishments in India. With a well-established presence in India, CliniExperts can help you comply with CDSCO requirements and start selling your device in this emerging market. CliniExperts hold a drug wholesale license in Form 20-B and 21-B and can be your in-country representative and IVD importing a hassle-free process.
Permission to import or manufacture new medical device in India
Importer | Regulatory Body: CDSCO
Get experts assistance to avail Permission to import or manufacture medical device which does not have its predicate device in India -As per MDR 2017
Related Services
Import License For In-Vitro Diagnostic Kits in India – Form MD14 & MD15
The license to import in-vitro diagnostic kits (IVD) is regulated in India under two regulatory provisions – Drugs and Cosmetics Act, 1940 and Drug and Cosmetics Rules, 1945. Import license for in-vitro diagnostic is received in “Form MD 15” as per Medical Device Rules, 2017.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Authorized Agent Support In-Vitro Diagnostic Kits
In India, the manufacturing, import, sale and distribution of medical devices are regulated under the Drugs and Cosmetics Act and Rules by The Central Drugs Standard Control Organization (CDSCO).
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Clarification Letter / No Objection Certificate for In-Vitro Diagnostic Kit
The Central Drugs Standard Control Organisation (CDSCO) is the Indian regulatory body that governs the safety, efficacy, and performance of medical devices. The CDSCO also provides clarity to importers and manufacturers regarding the regulatory status of the product.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Test License to Import In-vitro Diagnostics in India
The regulatory body of India has laid down provisions for non-notified medical devices. The products already under the notified category of the medical device are excluded from this category.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
IN-VITRO DIAGNOSTIC KITS LABEL COMPLIANCE
The registration process for medical devices is primarily done on the SUGAM portal, a website where the applicants apply for approval of licenses, FSC and Registration numbers.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Permission For Test License To Manufacture In-Vitro Diagnostic Kits (IVD) – (Form MD 12, MD 13)
A medical device under Class A, B, C, or D can be manufactured in small quantities for various purposes; for instance, a medical device can be used for clinical investigation, testing, evaluation, examination, and demonstration or training.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Permission to Manufacture Class C & D In- Vitro Diagnostics in India
For manufacturing an in-vitro diagnostic medical device (IVD) of Class C & D, a Manufacture license must be taken before starting the manufacturing process. Any manufacturer who intends to produce a device at a site where the same device is being produced by another manufacturer must get a Manufacture license.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Post Approval Changes For In-Vitro Diagnostic Kits
Get authorized agent support for all your post approval changes to In-Vitro Diagnostic Kits substance for already approved IVD manufacturing site.
Imoprter | Regulatory Body: CDSCO
ENQUIRE NOW
Permission for Loan License to Manufacture Class A & B In- Vitro Diagnostics in India
The Manufacturing License for sale and distribution of Class A and Class B In- Vitro Diagnostic Kits are regulated by the State Licensing Authority.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Free Sale Certificate For IVD
A free sale certificate, also known as a “Certificate for Export” is a document issued by the national regulatory authority. The Central Drugs Standards Control Organization (CDSCO) streamlines the application process for free sale certificate for notified In-vitro diagnostic kits in India.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Assistance in ISO 13485 Certification in India in India
The purpose of this service is to assist in ISO 13485 certification process. ISO 13485 Certificate is an important Certification required by the manufacturers, designers, suppliers, distributors, and service providers of all medical devices including in vitro devices (IVDs).
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Permission to Manufacture Class A & B In Vitro Diagnostics in India
The purpose of this service is to obtain license to manufacture Class A and B IVD’s in India. The State Licensing Authorit is the regulatory body associated with this service. It grants the license to manufacture IVDs for sale or distribution in FORM MD-5 as per Medical Device Rules, 2017.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Permission for Loan License to Manufacture Class C & D In- Vitro Diagnostics in India
A Loan manufacturing license in FORM MD-10 is needed by any manufacturer, as per provisions of the Medical Device Rules, 2017 for manufacturing the IVD’s of Class C & Class D (Notified) in India.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Registration For Non-Notified In Vitro Diagnostic in India
All non-notified In-vitro Diagnostic (IVDs) medical devices, excluding those already under the notified category of the medical device need registration with the CDSCO.
Importer | Regulatory Body: CDSCO
ENQUIRE NOW
Test License to Import In-vitro Diagnostics in India in India – MD 16 & MD 17
The Central Drugs Standard Control Organization (CDSCO) has issued a notice stating that a small number of medical devices might be imported into India. The devices belonging to Class A, Class B, Class C, or Class D may be imported in small quantities to India based on Test License in Form MD-17.
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW
Permission to conduct Clinical Performance Evaluation in India – MD-24 & MD-25
The Central Licensing Authority, CDSCO, is the regulatory body associated with this service. This service aims to obtain Permission to conduct a Clinical Performance Evaluation of all new in-vitro diagnostics (IVDs).
Manufacturer | Regulatory Body: CDSCO
ENQUIRE NOW

Insight
India Champions e-Governance for Pharma Industry
National e-Governance plan (NeGP), launched in 2006 as initiative that Government of India has implemented to make all government services accessible online.
Read More
Insight
Sugam Portal – CDSCO Sugam Registration for Online Application
Indian Government has chosen to join the foray and ride the digital wave through SUGAM, launched on 14 November, 2015
Read MoreRegulation/Guidelines
New Regulations for Medical Devices Industry by CDSCO
Nowadays, therapeutic treatment based on medical devices is providing technologically advanced solutions for the management, diagnosis, treatment,mitigation or prevention of several diseases. Thus, the demand continues to grow in the market at a tremendous rate leading to a renewed interest in the scientific development and research in the field of […]
Read MoreNews
Self-Assessment/Audit of Unit for GMP/GLP Compliance
The Indian government has brought about some major changes with regards to the rules and regulations governing the manufacturing to enhance the quality of products used in the healthcare industry. As India being a major market for the healthcare-related products and its services, these modifications to the existing regulations are […]
Read MoreFeature
Consultation on Medical Device and Diagnostic Kit for India Market
Import and Registration of Medical Devices, Registration certificate of Critical / Notified Diagnostic Kits, Test licence for Medical Device and Diagnostic Kits, Import License for Diagnostic Kits, Manufacturing licence for medical device, Clinical Trial Approval for Medical Devices and Diagnostic Kits
Read More