Permission to Manufacture Class C & D Medical Devices in India - Form MD 7 & MD 9

CliniExperts can function as authorized agent in India. It has a firm position in the Indian Healthcare market, and holds a valid Drug Wholesale License. CliniExperts has expert professionals who will provide all the support throughout the application process and submission of Form MD-7 for your manufacturing sale or distribution licenses for Class C and Class D medical devices.
Permission to Manufacture Class C & D Medical Devices (Form MD 7, 9) – Overview
The highest Indian regulatory body supervising the manufacturing of notified medical devices is the Central Drugs Standard Control Organisation (CDCSO). In India, the regulatory authority is responsible for giving license and approval for the manufacturing of medical devices. This regulatory authority provides the manufacturing licenses in Form MD-9 which is required as per the provision of Medical Device Rules, 2017.
An application needs to be submitted to the Central Licensing Authority for a manufacturer license. This can be applied through the online portal of the Central Government. A manufacturer license allows the sale or distribution of Class C and Class D medical devices in Form MD-9 which is obtained by applying via Form MD-7.

Who Can Apply?
The application for permission to manufacture Class C & D Medical Devices can be submitted by any person who wishes to manufacture these devices through Form MD-7.
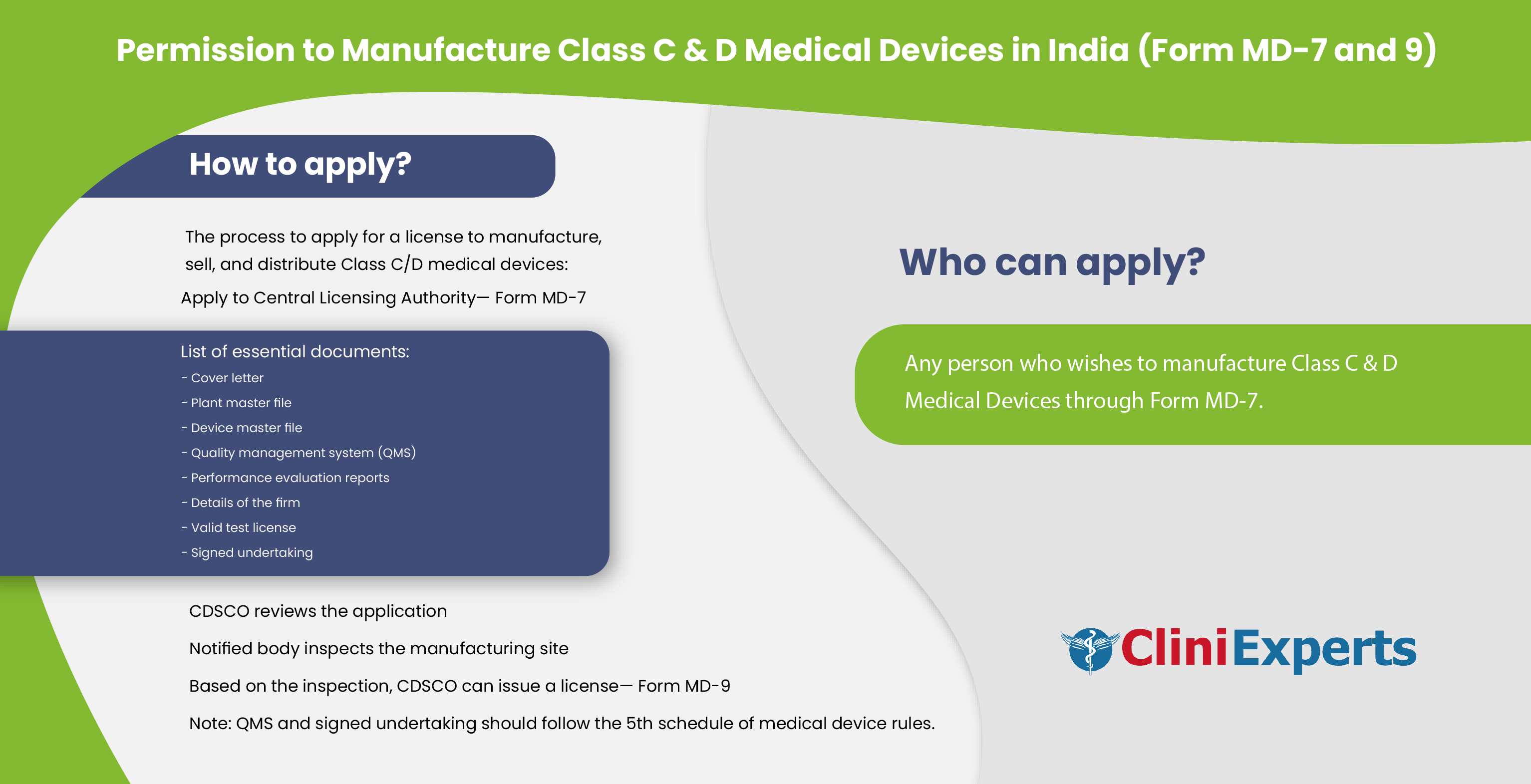
How To Apply?
The Applicant must follow the following process:
-

Application can be made to the Central Licensing Authority in Form MD-7
-

The application requires the following documents:
-

Cover letter, plant master file and device master file
-

Quality Management System specified as per the 5th schedule of Medical Device Rules and performance evaluation report (IVDs only)
-

Constitution detail of the firm, the establishment/site ownership/tenancy Agreement
-

Copy of Duly notarized valid copies of Quality Certificate in respect of manufacturing site
-

Valid Test License obtained for testing and generation of quality control data
-

Singed undertaking stating that the manufacturing site is following provisions of the Fifth schedule.

Validity
A valid Form MD-9 license does not have fixed expiry date; it lasts forever subject to the timely payment of license retention fee within 5 years of the issue date as per the Second Schedule, unless it is suspended/ cancelled by the Central Licensing Authority . Upon failure of payment on time, the license stands suspended/cancelled.

Fee Involved
The government charges fees for manufacturing. The fees involved for Class C or Class D for one site of manufacturing medical devices is 50000 INR whereas each distinct medical device cost is 1000 INR.Important Documents

The necessary documents required for the application with Form MD-7 are –
- Device Master File
- Site Master File
- QMS Documents
Timeline to get
MD 9
from CDSCO
Essential Tips
- For Class C and D devices, the manufacturing site of the applicant must be according to the requirements of the Quality Management System that are mentioned under the Fifth Schedule. The device master files and site master files are essential and need to be prepared as per the format given in MDR 2017 at the time of application.
- During the submission process, manufacturers can experience problems if the technical documents of the products are not filed according to MDR 2017. To avoid undue delay, these documents must be completely prepared as per the latest MDR guidelines.
Expert Advise
The quality control data is generated based on a valid Test License must be submitted during the application process for quick approval.
Related Services
SUGAM Registration- MD/ IVD
Importer | Regulatory Body: CDSCO
The registration process includes several steps to obtain approvals. The process involves registration, undertaking, and uploading the documents, complying with the given rules and regulations. We at CliniExperts, make sure that our expert team creates your registration easy and quick so that you can focus on more important aspects of your product launch...
Import License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Meet all your Regulatory Compliance needs. CliniExperts' professionals help you plan and streamline regulatory approval processes.
Test license to Manufacture Medical Devices in India – MD 12 & MD 13
Manufacturers | Regulatory Body: CDSCO
CliniExperts dedicated team helps in getting the Permission For Test License To Manufacture Medical Devices. We strategically plan the process for regulatory approval of manufacturing test license for medical devices in Form MD 13.
Free Sale Certificate from CDSCO for regulated Medical Devices in India
Importer | Regulatory Body: CDSCO
A Free Sale Certificate for Medical Devices is mandatory to import or export of medical devices to and from India. CliniExperts helps medical device companies secure A Free Sale Certificate or Certificate to Export medical devices.
Frequently Asked Questions
When does the CDSCO conduct the inspection of the manufacturing site of Class C& D Medical devices?
After the submission of the application, within sixty days CDSCO inspects the manufacturing site of the Class C& D Medical devices.

How many members of the Audit team are prescribed in MDR 2017?
A team comprising of minimum two medical device officers, one could be any officer senior to the medical device officer, with or without an expert, or a Notified Body.

What is the timeline for carrying out the inspection for class C & D and the grant of license?
The Central Licensing Authority conducts the inspection for class C and class D within a period of 60 days from the date of application. After complete satisfaction, Central Licensing Authority may grant a license within a period of forty-five days from the date the inspection report is received.

The fourth schedule specifies that the manufacturers have to submit an undertaking that they comply with the provisions of the Fifth schedule. Is this also applicable for a license to manufacture?
Yes. The undertaking signed stating that the manufacturing site is in compliance with the provisions of the Fifth Schedule of MDR 2017 needs to be submitted in case of manufacture of Class C and D medical devices.

If a manufacturing firm is complying with ISO/IEC standards, would it still need to follow benchmarks laid by Bureau of Indian Standards (BIS)?
Yes. The medical device must obey the standards laid down by the BIS established under section 3 of the Bureau of Indian Standards Act, 1985 (63 of 1985) or as may be notified by the Ministry of Health and Family Welfare in the Central Government, from time to time. If no relevant standard of any medical device has been laid down under sub-rule 1, such device shall follow the standard laid down by the International Organization for Standardization (ISO) or the International Electro-Technical Commission (IEC), or by any other pharmacopoeia standards. In case of the standards which have not been specified under sub-rule (1) and sub-rule (2), the device shall follow the validated manufacturer’s standard.

What would be the transition timeline given to manufacturers w.r.t grandfathering of already existing devices?
If the device has already been marketed and the Government of India notifies the same under 3(b)(iv) of the Drugs and Cosmetics Act, 1940 (23 of 1940), then the device will be regulated under the Medical Device Rules, 2017.

For devices that are already in the market and notified later, would the requirement of local clinical investigation/evaluation be waived off?
Each medical device will be deliberated on a case-to-case basis, on the basis of their intended use, and with data available, to substantiate their safety and effectiveness. The matter may also be placed before Subject Experts Committee (SEC).

