Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
July 28, 2016
As per the new regulations under rule 122 DD, CDSCO has made it mandatory for all Ethics committee to get registered with CDSCO so as to get authority to review and accord...

Blog
July 28, 2016
“DCGI has made it mandatory for all manufacturers and importers who have obtain new drug permission to submit Periodic Safety Update Reports of new drug every six months for the first two year and...

Blog
July 28, 2016
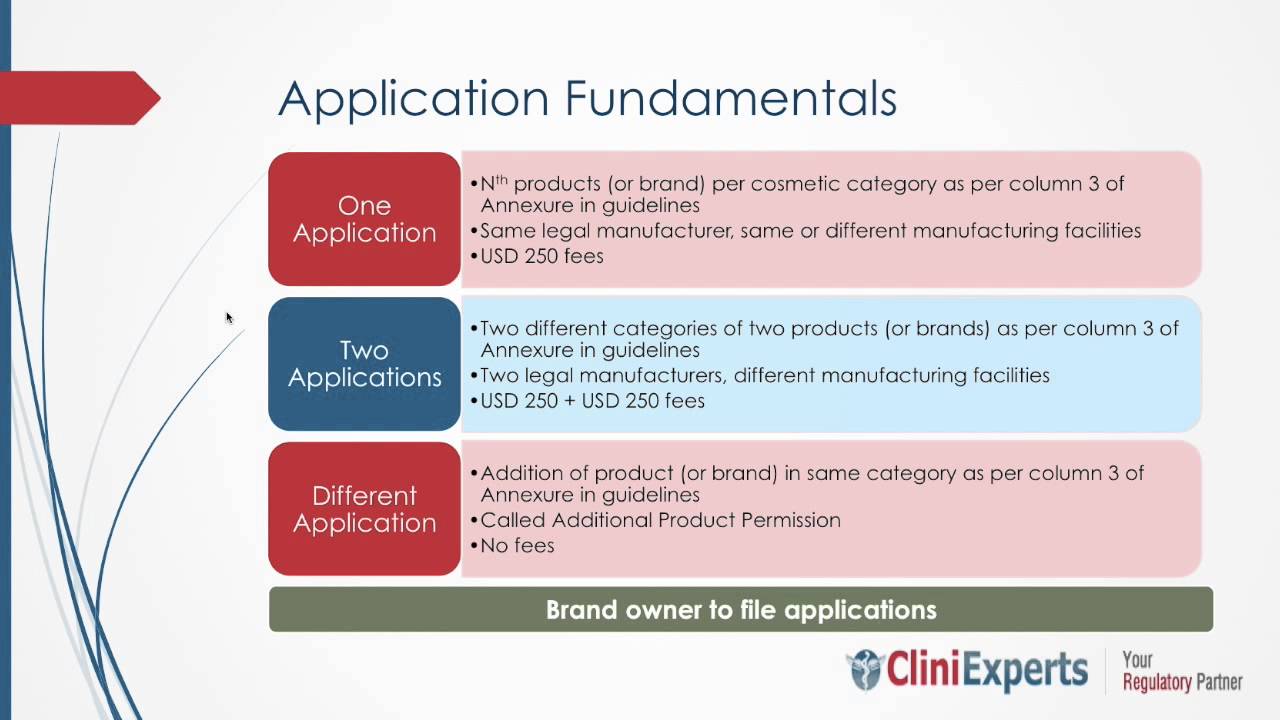
No cosmetic shall be imported into India unless the product is registered under the rules by the licensing authority appointed by the Central Government under rule 21 or by any Person to...

Blog
July 27, 2016
CliniExperts is a leading cosmetic regulatory consultant based in New Delhi, India. The cosmetic regulatory strategy consulting & liaison services are provided by the expert staff at CliniExperts at a...

Blog
July 27, 2016
Are you looking to get new cosmetic products application, submission & approval in India? Your search end here… Any cosmetic product before reaching the consumer’s hand has to go through...

Blog
July 27, 2016
The cosmetic marketing in India and the cosmetic import regulation in India are regulated and the government of India has issued a Gazette Notification G.S.R 426(E) dated 19th May 2010 for amending...

Blog
July 27, 2016
An Expert Approach To Resolve Technical And Non-Technical Issues Regarding Cosmetic License And Cosmetic Registration Throughout India CliniExperts offers one stop solution for cosmetic license application & registration in India. We...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding