Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
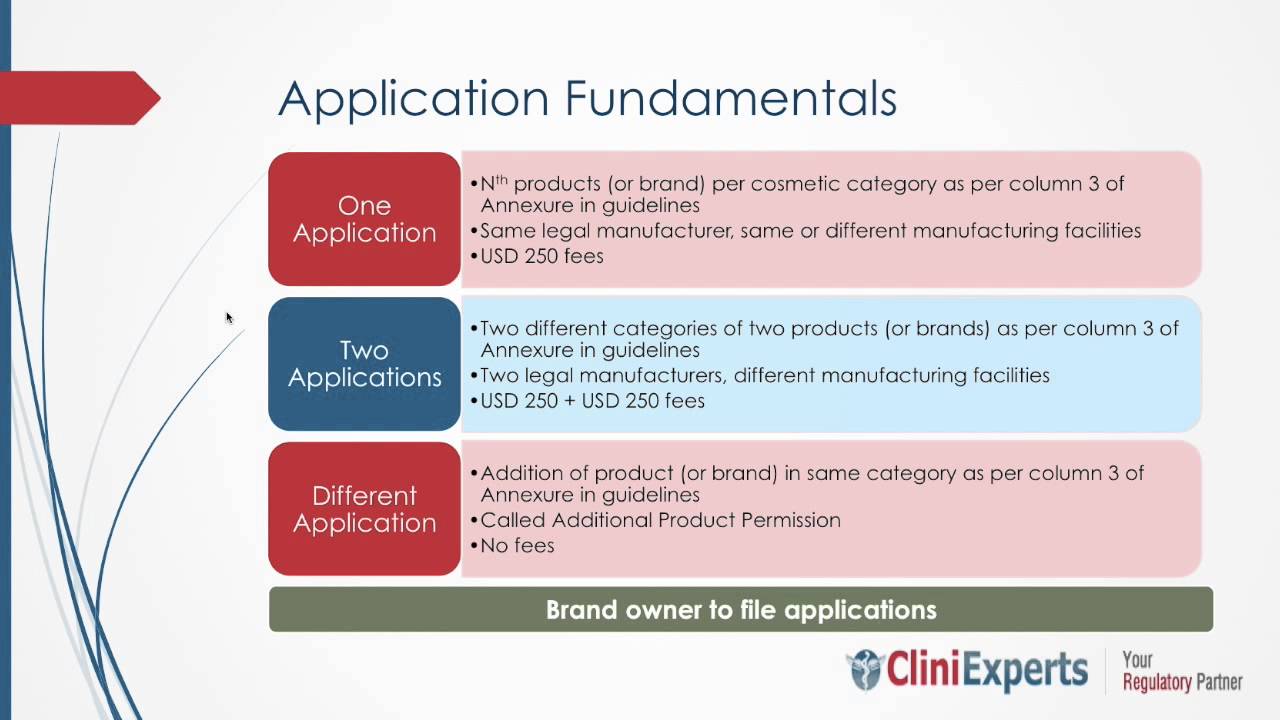
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
July 12, 2016
Leading FSSAI License Consultant in India CliniExperts facilitates speedy product approval from FSSAI to gain fast-paced entry into the lucrative Indian food market. Intricacies of food compliance in India FSSA, 2006 stipulates...

Blog
July 12, 2016
We expertise in preparationof: Manuscript Writing / Editing Conference Abstracts / Presentations Content, Design and Layout of Scientific Posters Journal Selection / Submission Editorial Writing Product Monograph Patient Information Leaflet Summary of...

Blog
August 10, 2014
The requirement of local trial for a generic or similar biological (Bio- Similar) in other country like USA for its approval in the country, it has been decided that the drugs considered...

Blog
August 8, 2014
Health minister Dr Harsh Vardhan stated that food business operators (FBO) have to adhere to the advisories and guidelines that have been issued by the Food Safety Standards Authority of India (FSSAI)...

Blog
August 5, 2014
The Food Safety and Standards Authority of India (FSSAI), the country’s apex food regulator, has extended the deadline for obtaining licences and securing registration for food business operators (FBOs) in the country...

Blog
July 10, 2014
By Jill Hartzler Warner, J.D. The U.S. Congress and the Food and Drug Administration have long focused on bringing new therapies to patients with rare diseases, including children. Two years...

Blog
July 10, 2014
It has been decided by the Expert Committee constituted by the Ministry of Health & Family Welfare that waiver of Clinical trial in Indian population for approval of new drugs, which have...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding