Update on Food Safety and Standards (Labelling and Display) Regulations Draft, 2018

The Food Safety and Standards Authority of India on April 11, 2018 has published the Draft for Food Safety and Standards (Labelling and Display) Regulations, 2018. These regulations propose to prescribe the labelling requirements of pre-packaged foods and display of essential information on premises where food is manufactured, processed, served and stored.
This draft has made it mandatory for FBOs to declare foods as genetically-engineered (GE) or modified (GM) for all food products having five percent or more genetically-engineered ingredients. The total GE ingredients shall be of the top three ingredients in terms of their percentage in the product. The labelling shall read, “Contains genetically-modified organisms (GMO)/Ingredients derived from GMO”.
FSSAI has also defined high fat, sugar and salt (HFSS) foods as processed food products having high levels of total fat or trans-fat or total sugar or salt.
According to the draft, the following information shall be declared on the front portion of the pack: “Name of the food product; declaration regarding vegetarian or non-vegetarian; per serve contribution of energy, total-fat, trans-fat, total sugar and salt (sodium chloride) to the recommended dietary allowance (RDA) as per the format indicated below:
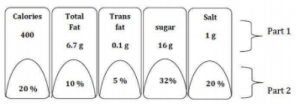
Part-I: Declares the amount of energy, total fat, trans fat, total sugar and salt (sodium chloride) per serve;
Par-II: Declares the per serve percentage contribution to RDA as provided under Regulation 4.2 (3) (b);
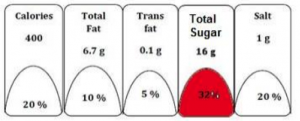
The block(s) of nutrient(s) for high fat, sugar and salt (HFSS) food shall be colored red as depicted above, in case the value of energy (kcal) from total sugar is more than 10% and from trans-fat is more than 1% of the total energy (kcal) provided by 100g/100ml of the product; Also, threshold values for energy (kcal) from total fat or sodium content provided by 100g/100ml of the product have been specified in Schedule–I.
According to the draft, nutritional information per 100g or 100ml of the product and per serve percentage contribution to RDA (calculated on the basis of 2,000 kcal energy, 67g total fat, 2g trans-fat, 50g total sugar and 5g salt (sodium chloride) requirement for the average adult per day), shall be given on the label containing the following information:
- Energy value (kcal);
- The amounts of
- Protein (g);
- Carbohydrates and sugars (g);
- Total fat (g), saturated fat (g), trans-fat (g) and cholesterol (mg); Provided that the amounts of saturated fat and trans-fat may be declared on the label as “not more than”.
- Salt (sodium chloride)
FSSAI explained that serving or serve size means an amount of food customarily consumed per eating occasion or as defined on the label which is expressed in metric unit. Additionally, it may also be mentioned in common household measures like teaspoon, tablespoon and cup whichever is appropriate for the respective food.
However, food for catering, hospitals, restaurants and food service vendors are exempted from this labelling along with food made of single ingredients like sugar, jaggery, salt, spices, non-nutritive products like coffee, tea and packaged fresh fruits and vegetables, seafood, etc.
The draft also specifies the following conversion factors, to be used to calculate the amount of energy to be listed on the pack:
(A) Carbohydrates: 4kcal/g
(B) Polyols except erythritol: 2kcal/g
(C) Erythritol: 0kcal/g
(D) Protein: 4kcal/g
(E) Fat: 9kcal/g
(F) Alcohol (ethanol): 7kcal/g
(G) Organic acid: 3kcal/g
“Nutritional information may additionally be provided in the form of bar code/global trade identification number (GTIN),” as proposed in the draft.
For vegetarian products, a green color-filled triangle inside a square with a green outline, has also been prescribed for the FBOs

Whereas, a brown color-filled circle inside a square with a brown outline for non-vegetarian products with specified size.

Further the draft also prescribed a black-colored cross inside a square with black outline, for food material not meant for human consumption.

The draft also says that the declaration regarding the food product to be vegetarian or non-vegetarian shall also be prominently displayed as mentioned in this regulation, on the pamphlets, leaflets and advertisements in any media, for the food product.
Regarding the date marking, the draft mentions that, “The date of manufacture or packaging and expiry/use by shall be declared on the label. Besides, the expression best before may also be used as optional or additional information.
FSSAI also expresses, “That In case of imported food, the country of origin of the food shall be declared on the label of food imported into India. When a food undergoes processing in another country which changes its nature, the country in which the processing is performed shall be considered to be the county of origin for the purposes of labelling”.
The draft also stated that the area of the principal display panel shall not be less than 40 per cent of the total package area in case of rectangular and cylindrical packages while 20 percent for other shapes. However, in case of a package having a capacity of 10 cubic centimeters or less, the principal display panel may be a card or a strip of tape affixed firmly to the package and bearing the required information.
For the packaging of fortified food, label should carry the words “fortified with …… (name of the fortificant) and the specified logo. It may also carry a tagline “Sampoorna Poshan Swasth Jeevan”. In case of organic products, the label shall carry the logo of Jaivik Bharat.
The FBOs needs to label their product, according to the new norms prescribing the details once the draft is accepted and passed into a final regulation.
For more insights and help on labelling regulations, please feel free to reach us at contact@cliniexperts.com
REFERENCE
- Draft on Food Safety and Standards (Labelling and Display) Regulations, 2018. F.No 1-94/FSSAI/SP(Labelling)/2014(Pt-2). FSSAI. April 11, 2018
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


