What are CDSCO Form MD 26 and 27 and the benefits of having the import license for new medical devices?

An import license for new medical devices (Form MD 26 & MD 27) is a prerequisite for starting a business to sell or distribute medical devices. Having an import license gives a clear idea about the specification and standards of imported Medical devices.
It also guarantees the compliance of Medical devices with Quality Management System standards
Thus, it is advisable to have a proper import license due to the strict rules and regulations of the regulatory authority of India.
The import license allows the government to know the quality of Medical Devices so, the customers know that it is safe to use and is trustworthy.
Import license-
- Any person/firm/enterprise etc., who holds a wholesale license or manufacturing license issued under the Drugs and Cosmetics Act, 1940 and Rules 1945, can apply for Registration and import of medical devices into India.
- No import license is needed, only a valid manufacturing license is required to import raw materials/components intended to be used for further manufacture of Finished Medical Devices.
- Under chapter 5 legislation, an authorized agent who holds a manufacturing or wholesale license to sell or distribute medical devices is a prerequisite for getting the import license.
- As per the Medical Device Rules 2017, the regulatory authority of India Central Drugs Standard Control Organization (CDSCO), has Central Licensing Authority (CLA) which is responsible to, grant permission to import new medical devices in India.
- The application for permission to import/manufacture a new Medical Device can be made to the Central Licensing Authority through an online portal of the Ministry of Health and Family Welfare in the Central Government.
Benefits of Import License for New Medical Devices-
An import license for new medical devices has many benefits, which can help the manufacturer or importer. The person can sell the Medical Device without any legal problems, and it also ensures the safety of the intended population.
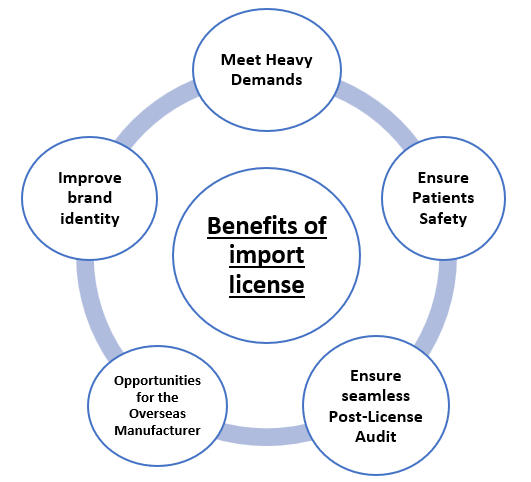
- Ensure Patients Safety –
- Are free from vulnerabilities or harmful health effects.
- When a CDSCO certifies a medical device, it is tested at the certified lab, has a quality certificate and delivers the best results depending on the class they represent.
- The tests on these products are supervised by authorities which often take months to approve them.
- With registered Medical Devices as the finished product, the safety and health effects of such types of equipment are not a concern
- Meet Heavy Demands –
- India is a country of high demand for medical devices, thus to meet those demands, the Indian market relies on overseas manufacturers.
- Thus, the regulatory body is not taking chances with the quality of the product.
- This is the reason, that the regulatory body was set up to correct moderate and poor-quality products.
- Improve brand identity –
- With new norms and regulations in place, every manufacturer has opportunities to explore the market dynamically after getting their product registered under a given law.
- It does not make sense to ignore the guideline for the sake of profit since the penalties are too steep to handle.
- Opportunities for the Overseas Manufacturer –
- The higher demand for medical devices in India pushes the Indian government to allow overseas manufacturers to import their medical devices.
- CDSCO allows overseas manufacturers to import their medical devices after complying with prescribed guidelines.
- Opportunities for the Overseas Manufacturer –
- To ensure whether Medical Devices are being manufactured and stored as per compliance the authority conducts on-site audits.
- The audit helps to identify the faults in equipment, helps to overcome the risks which can affect the patient in the future.
- So, keeping up with the compliances must be the priority of every manufacturer and distributor.
Difference between forms 26& 27-
Form MD 26
- Medical devices which do not have a predicate medical device in India need permission to import or manufacture medical devices i.e., it’s a type of application form.
Form MD 27
- Permission to import or manufacture for sale or distribution of new medical devices which do not have a predicate medical device. i.e., a type of permission form.
Summary –
Any person who wants to import/manufacture Medical devices in the Indian market needs to have an import/manufacturing license issued under the Drugs and Cosmetics Act, 1940 by the regulatory authority of India CDSCO.
CLA is responsible for the import of all the classes of MD. Form MD 26 is for getting permission from CLA either for manufacturing/importing Medical devices which do not have predicate devices in India, while permission is granted through Form 27. In this blog, you will know the several benefits of an import license.
- A permission in Form 26/27 has plentiful benefits for Importers/manufacturers who want to import/manufacture and sell medical devices which do not have predicate devices in India. Since this permission is under the control of the regulatory authority of India CDSCO, opens the Indian market for foreign manufacturers along with local manufacturers.
- More than 75%, Medical Devices are imported into the country thus, a large part of this sector depends on the import.
- In 2020-21, $6.24 billion worth of medical devices were imported by India, up from $5.84 billion in 2019-20 and $5.7 billion in 2018-19.
Saurangi is a food regulatory expert with 8 years of experience. She shares her knowledge and insights on regulatory updates, food trends, best practices, and news. Follow her for expert insights and practical advice on all things for food regulatory
Saurangi Shah
CliniExperts Services Pvt. Ltd.
Recent Posts
Organic Food Labelling In India| Certification, and Import of Organic Food in India

This Article is All About Organic Food Labelling In India and Certification, and Import of Organic Food in India. Explained in Detail About What is Organic Food labelling? Summary Short Description Wi..
Cosmetic Label Compliance India : A Guide to Compliance

Introduction Looking for Cosmetic Label Compliance India? Are you a cosmetic manufacturer or importer navigating the complex world of Indian regulations? Ensuring your product labels comply with the l..
Clinical Investigation Approvals: An Overview of Forms MD-22 and MD-23

Summary Short Description Strict regulatory protocols govern clinical investigations for medical devices. Central to this process are forms MD-22 and MD-23. Form MD-22 is an application to Central Lic..
HAVE A QUERY?
REACH US!Office
New Delhi
Unit No. 324 & 325, City Centre Mall, Plot No. 5, Sector 12, Dwarka, India - 110075
+917672005050
Bengaluru
RMZ Galleria, 1st floor, Ambedkar Colony, Yelahanka, Bengaluru, Karnataka, India – 560064
Call us on
Sales: +91 7672005050
Reception: +91-11-45214546
Timings
9 am to 6 pm (Monday to Friday)


