

Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
September 17, 2021

The Drugs and Cosmetics Act, 1940, and Rules regulate the safety, quality, and performance of all medical devices. The Central Government has notified Medical Devices Rules, 2017 vide G.S.R. 78E dated January...

Blog
May 31, 2021

Regulations for the import or manufacture of medical devices The Ministry of Health & Family Welfare, Government of India, has notified the list of equipment or devices which will be regulated as...

Blog
April 22, 2021

CDSCO has designated the National Institute of Biologicals (NIB), Noida, as Central Medical Device Testing Laboratory as per rule 19 of Medical Device Rules, 2017 released on 1st June 2018.NIB performs tests...

Blog
March 10, 2021
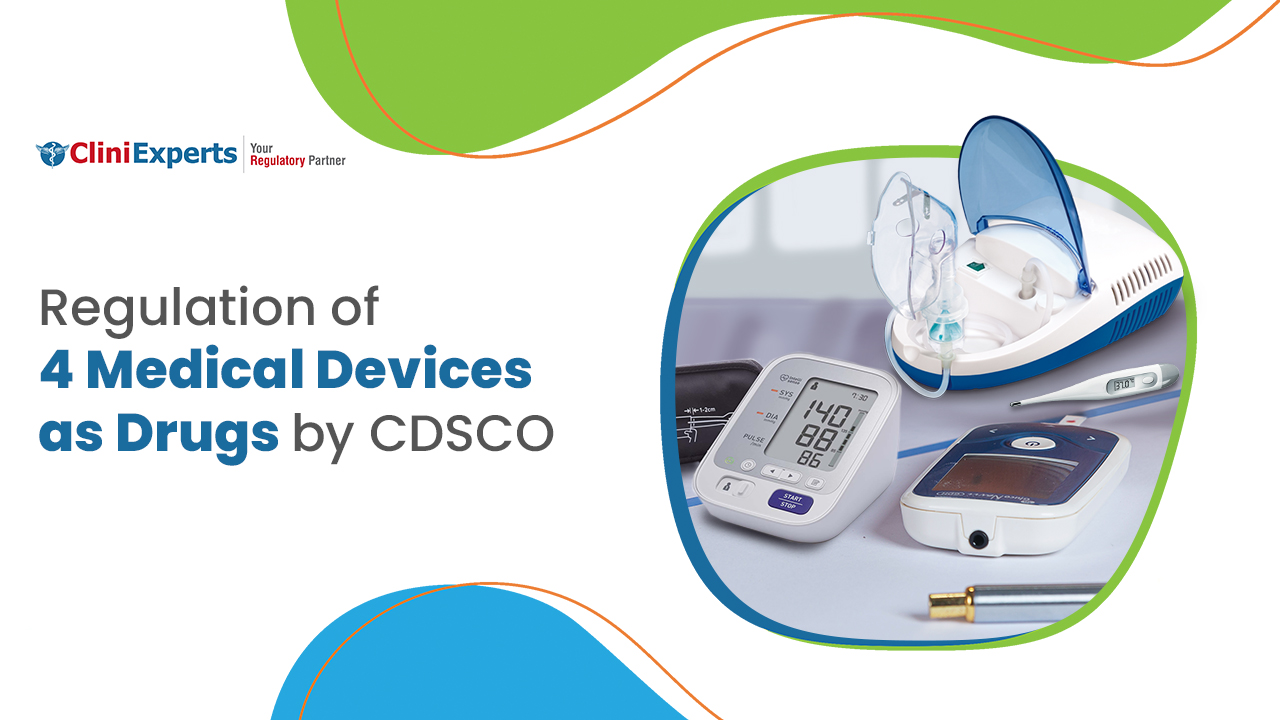
CDSCO has notified four medical devices, i.e., nebulizer, blood pressure monitoring devices, digital thermometer, and glucometer as drugs. The importers and manufacturers of these 4 medical devices need to take import and...

Blog
January 5, 2021

The Ministry of Health and Family Welfare has recently come up with a release of notification dated 21st October 2020 to extend the timeline from 1st day of November, 2020” to the...

Blog
November 9, 2020

The Central Drugs Standard Control Organisation (CDSCO) has released two notices on September 3, 2020, including the classification of non-notified medical devices and in-vitro diagnostic devices (IVDs). To simplify the classification process...

Blog
October 19, 2020

On 3rd September, 2020, CDSCO released a draft classification for non-notified Medical devices. The Ministry of Health and Family Welfare (MoHFW) is going to classify non-notified medical devices like gowns, adhesive drapes,...

Blog
September 18, 2020

Presently in India, only notified medical devices are regulated as Drugs under the Drugs and Cosmetics Act 1940 and Rules in 1945. The Central Drugs Standards Control Organization (CDSCO) streamlined the process...






