Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
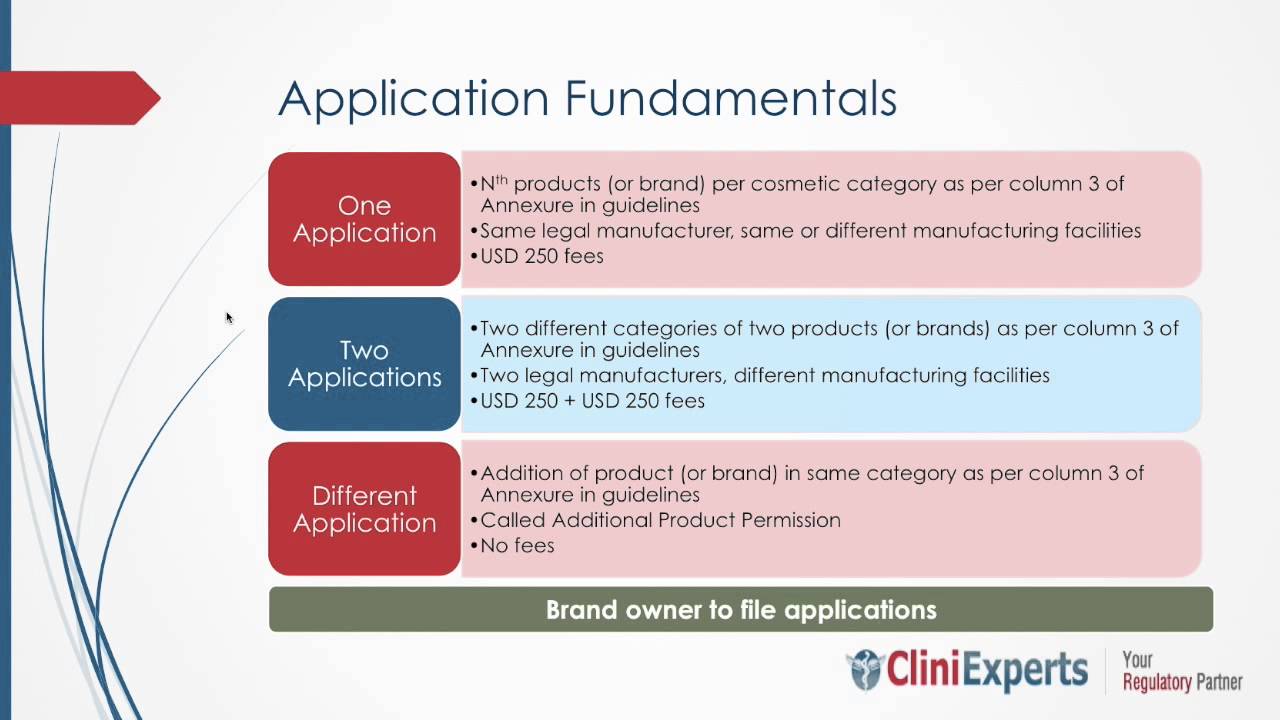
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
May 2, 2023

As per the order dated 10th October 2022, FSSAI notified that registration of Foreign Food Manufacturing Facilities (ReFoM) intending to export Egg powder; Nutraceuticals; Meat & meat items, including fish, poultry & their...

Blog
April 28, 2023

Active pharmaceutical ingredient (API) is defined as the component of a drug that is active biologically and is present in the tablet, injection, capsule, or cream and produces the intended health effect....

Regulatory Update
April 28, 2023

Regulatory Update
April 18, 2023

Blog
April 17, 2023

Drug Technical Advisory Board (DTAB): The Drug Technical Advisory Board is a committee constituted as per the provisions of the Drugs and Cosmetics Act, 1940. Drug Technical Advisory Board is a part...

Regulatory Update
April 11, 2023

Blog
April 4, 2023

FSSAI is an autonomous statutory body established by the Ministry of Health & Family Welfare, Government of India. FSSAI works under the regulations of the Food Safety and Standards Act 2006. It...

Regulatory Update
March 18, 2023
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding