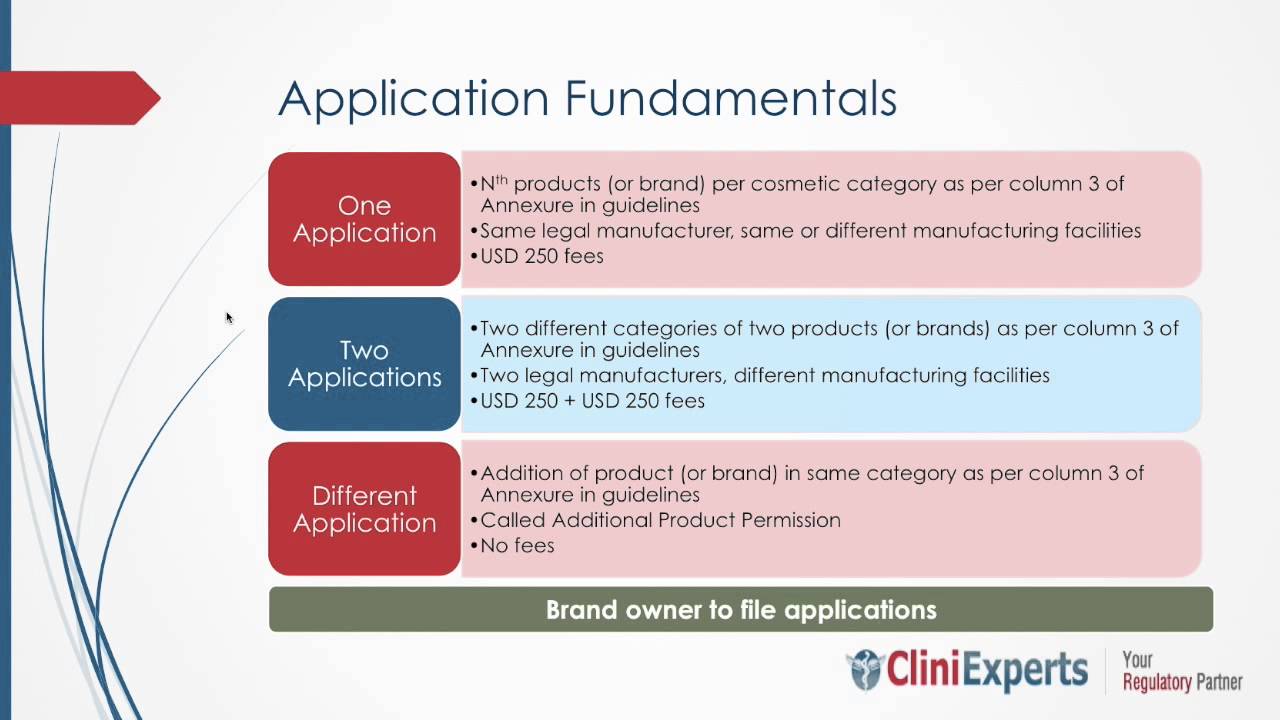
Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
June 16, 2022
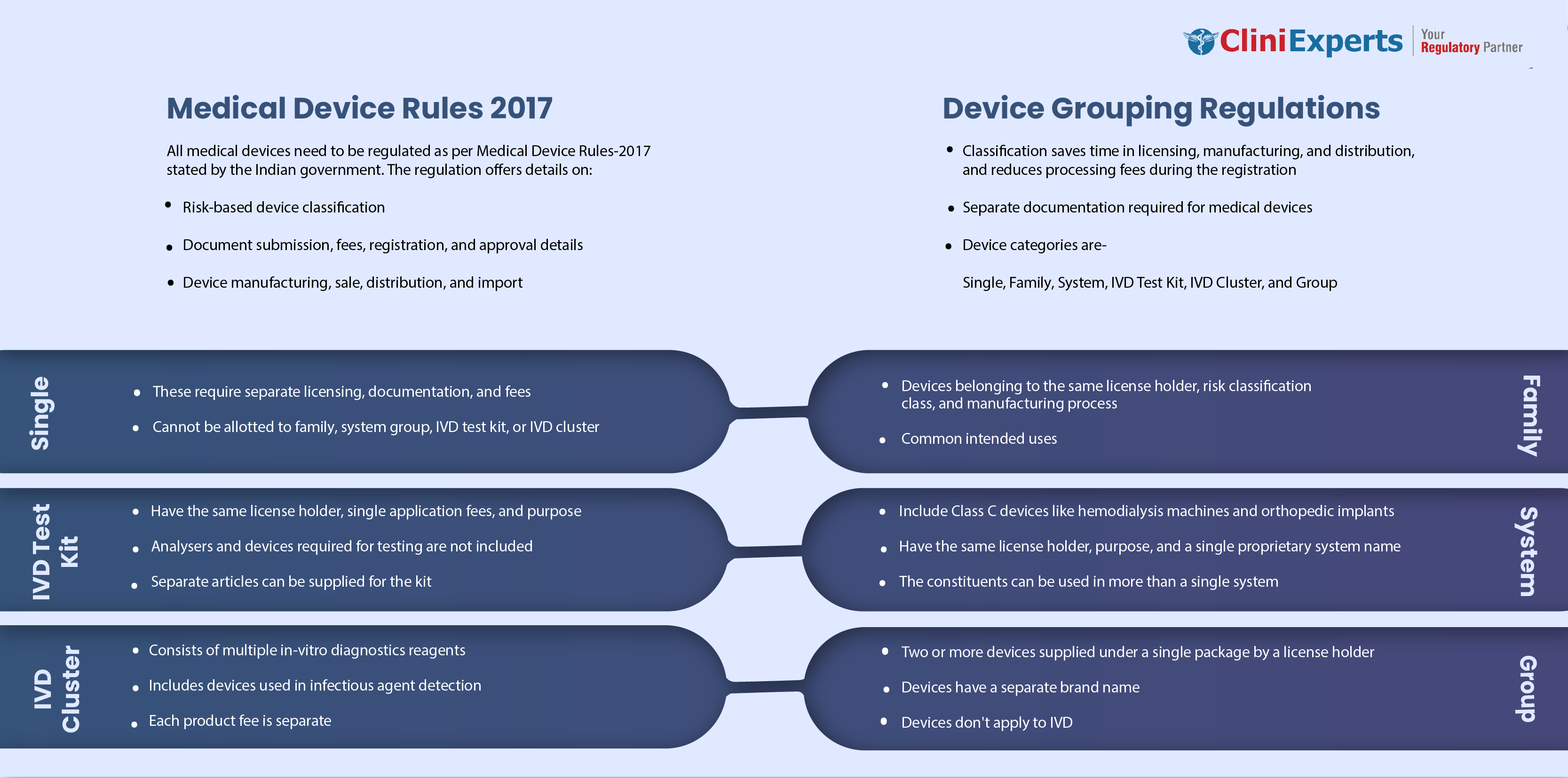
In the year 2017, the government of India announced that all the medical devices in India would be regulated as per Medical Device Rules-2017 (MDR-17), which gives a clear idea about the...

Blog
May 13, 2022
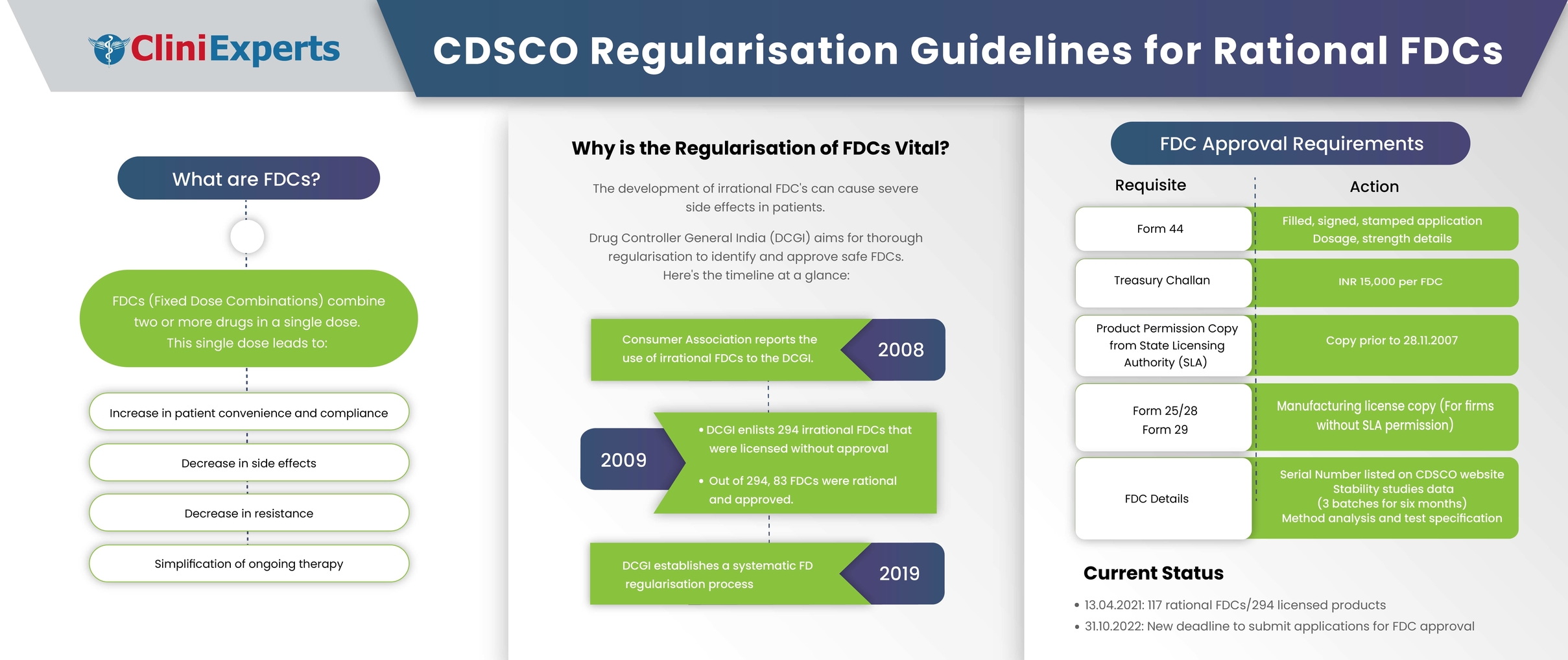
Rational fixed dose combinations (FDCs), also known as combination products, are the integration of two or more drugs in a single dosage form. The Food and Drug Administration (FDA), USA defines a...

Blog
April 14, 2022

What Is A Medical Device Authorized Agent / Representative? To register/sell a medical device in India, a Foreign Manufacturer must grant a Power of Attorney to a person/company in India who is...

Blog
April 4, 2022

According to the Drugs and Cosmetics Act, 1940, and New Cosmetics Rules 2020 and the Rules enacted thereunder, the State Drug Control Department inspects and grant cosmetic license approval for importers and...

Blog
March 22, 2022

An import license for new medical devices (Form MD 26 & MD 27) is a prerequisite for starting a business to sell or distribute medical devices. Having an import license gives a...

Blog
March 8, 2022

Wholesale Drug License In India - Overview The pharmacy business in India is booming. To ensure consumer safety, the Government keeps changing guidelines that aid streamlining of the processes as well. To...

Regulatory Update
February 28, 2022

Regulatory Update
February 28, 2022
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding