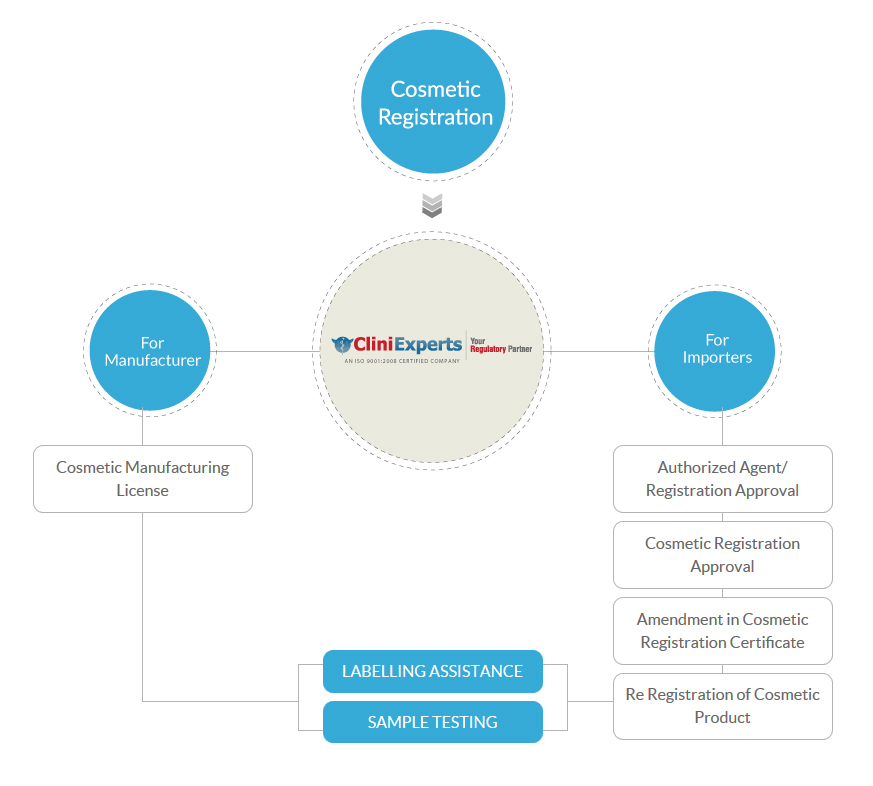
Cosmetic Registration
The cosmetic registration process requires meticulous planning and review of the ingredients and labeling for cosmetic registration in India. Any company intending to import and market cosmetic products has to go through this tedious process. The cosmetic application should clearly define the products in respective categories. With years of experience behind us in assisting several product registrations and certifications, we are the regulatory partner you need with you through the entire process in completing the cosmetic product registration without any glitches.
- For Manufacturer
- Cosmetic Manufacturing License
- For Importers
- Authorized Agent/ Registration Approval
- Cosmetic Registration Approval
- Amendment in Cosmetic Registration Certificate
- Re Registration of Cosmetic Product
For Manufacturer
We offer one stop solutions for cosmetic manufacturers helping them obtain the manufacturing license and test their products from certified labs in India. The following licenses are required for cosmetic product manufacturing & market in India according to D & C Act, 1940.
- License in Form Cos-8 is issued for manufacturing cosmetics for sale or for distribution. (Application is filed in Form Cos-5)
- License in Form Cos-9 is issued for loan license for manufacturing cosmetics for sale or for distribution. (Application is filed in Form Cos-5)
- License on Form Cos-23 is issued for grant or renewal of approval for carrying out tests on drugs/cosmetics or raw material used in the manufacture thereof on behalf of licenses for manufacture for sale of drugs/cosmetics. (Application is filed in Form Cos-22).
For Importer
CliniExperts helps to make the foray into the Indian market safe, hassle free and well planned. There are many challenges and legal red tapes often faced by companies who are foraying into the cosmetic business in India be it an importer, trader or marketer etc.
CliniExperts hand holds our clients as one of the most seasoned regulatory service provider in the country and guides cosmetic companies with all details and formalities, assisting them in clearing off all paperwork hitch free.
We act as an authorized agent or Indian representative on behalf of our foreign clients who are planning to import cosmetic products to India and help them obtain registration certificate.
Strategic planning and meticulous documentation by the CliniExperts team ensures hassle-free cosmetic registration and approval in India. Cosmetic Product Registration Certificate is obtained in Form Cos-2 for Cosmetic products.
For amendment in Registration Certificates, application with appropriate documents needs to be filed at CDSCO. This process is handled by our team of professionals smoothly.
We file the re-registration applications beforehand on behalf of our clients.
Labeling Assistance
Cosmetic Labeling is required to be done as per the regulations set by the CDSCO. CliniExperts makes sure that all information regarding RC number, font format, manufacturing details, etc. are available on the product label prior to marketing.
Cosmetic Sample Testing
The cosmetic product should be tested to comply with the standards as required. Through CliniExperts, cosmetic sample testing can be done through certified labs across the country to ensure all standards required are met.
Related Services
Wholesale Drug License in India
Importer | Regulatory Body: SLA
CliniExperts offer strategic planning services for Wholesale Drug License to start with your pharmaceutical business and sell drugs at distributor level. Our hand in glove approach ensures that at no point you find yourself battling with any process by yourself.
Clarification Letter / No Objection Certificate for Medical Devices in India
Importer | Regulatory Body: CDSCO
Unclear about the regulatory status of Medical Devices in India. Let CliniExperts’ professionals assist you for getting a Clarification Letter / No Objection Certificate (NOC) for Medical Devices from Central Drugs Standard Control Organisation.
Test License for Medical Devices in India
Importer | Regulatory Body: CDSCO
Need a permission to import medical device in India to demonstrate its performance? CliniExperts’ professionals have expertise and assist you in securing a medical devices test license for importers in Form MD 17 by CDSCO.
Non-Notified Medical Devices Registration/ Approval in India
Importer | Regulatory Body: CDSCO
CliniExperts acts as an authorized representative to help you in getting the permission to import non-notified medical devices in India. Register your Non-notified Medical Devices in India with CliniExperts professional assistance
Authorized Agent For In-Vitro Diagnostic Kits
Importer | Regulatory Body: CDCSO
Importing an in-vitro diagnostic kit and selling it across the country can be overwhelming if you do not have any local establishments in India. With a well-established presence in India, CliniExperts can help you comply with CDSCO requirements and start selling your device in this emerging market. CliniExperts hold a drug wholesale license in Form 20-B and 21-B and can be your in-country representative and IVD importing a hassle-free process.
Permission to import or manufacture new medical device in India
Importer | Regulatory Body: CDSCO
Get experts assistance to avail Permission to import or manufacture medical device which does not have its predicate device in India -As per MDR 2017
Insight
Sugam Portal – CDSCO Sugam Registration for Online Application
Indian Government has chosen to join the foray and ride the digital wave through SUGAM, launched on 14 November, 2015
Read MoreFeature
Cosmetic Regulatory Strategy Consulting & Liaison Delhi, India
Cosmetic regulatory strategy consulting & liaison by cosmetic regulatory consultant based in New Delhi, India
Read MoreFeature
Cosmetic Products Submission & Approval in Delhi, India
Are you looking to get new cosmetic products application, submission & approval in India? Your search end here
Read MoreInsight
Cosmetic Products Marketing & Import Regulation in India
Registration of Import of cosmetics and Marketting in India
Read MoreInsight
Cosmetic License Application & Registration in India
An expert approach to resolve technical and non-technical issues regarding Cosmetic License and Cosmetic Registration throughout India
Read MoreInsight
Cosmetic Regulations, Registration and Import Registration in India
Description on the Regulation and Registration in India for Imported Cosmetics
Read MoreNews
Self-Assessment/Audit of Unit for GMP/GLP Compliance
The Indian government has brought about some major changes with regards to the rules and regulations governing the manufacturing to enhance the quality of products used in the healthcare industry. As India being a major market for the healthcare-related products and its services, these modifications to the existing regulations are […]
Read MoreStrategy
Import NOC for import of small quantity of cosmetics
CliniExperts Services can assist the wholesale traders or manufacturers interested in importing small quantity of cosmetics into India for R&D and testing purpose
Read MoreInsight
Cosmetics Registration
No cosmetic shall be imported into India unless the product is registered under the rules by the licensing authority appointed by the Central Government under rule 21 or by any Person to whom such powers may be delegated under rule 22.
Read More



