Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
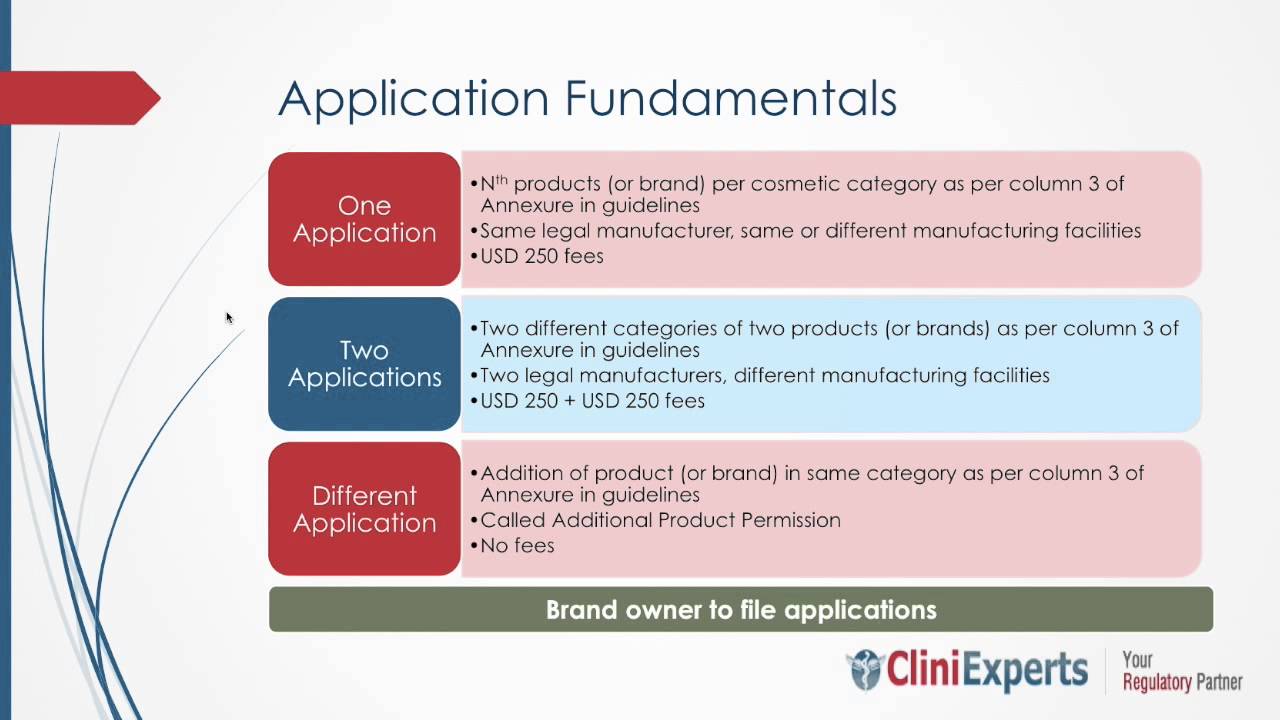
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Regulatory Update
August 19, 2022

Regulatory Update
August 19, 2022

Blog
August 18, 2022

ISO 14385 Medical Devices Certification: Definition ISO 13485 Medical Devices certification, Medical Devices-quality management system (QMS) is an internationally agreed standard. It sets out the regulatory requirements for quality management systems specific...

Blog
August 5, 2022

India's central consumer protection body published rules for preventing misleading commercials and endorsements for misleading advertisements 2022 on June 9th, 2020. CCPA announced the Guidelines to fulfill the duties given to CCPA...

Blog
July 28, 2022

The new draft bill consists of a few new definitions such as clinical trials, over-the-counter drugs, manufacturers, cosmetics, medical devices, new drugs, bioavailability, investigational new drugs, imported spurious drugs, predicate devices and...

Blog
July 21, 2022

Class B Medical Devices / In-Vitro Diagnostic Kits Class B medical devices have low to moderate risk to the patients and public health risks. Class B medical devices’ permissions or approvals are...

Regulatory Update
July 21, 2022
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding