Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
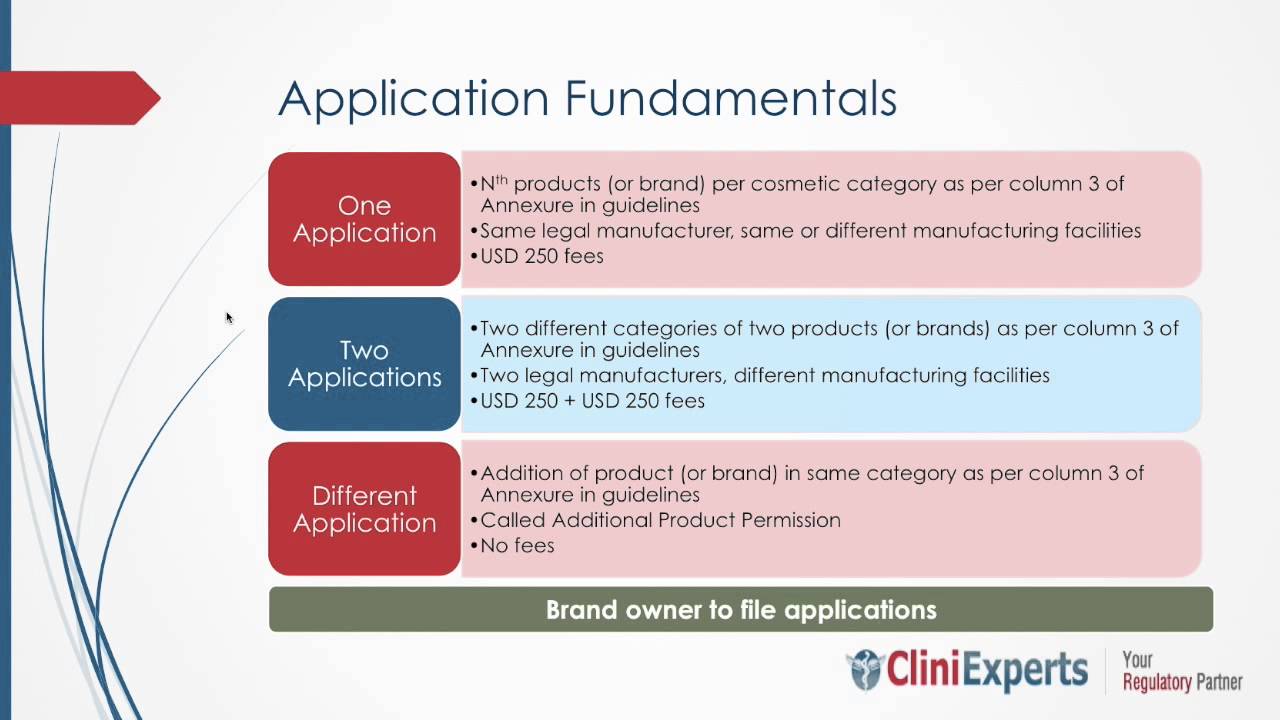
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
January 20, 2017

Millions of people consume caffeinated or carbonated beverages like cola and energy drinks every day. Adolescents and young adults are taking energy drinks in order to increase endurance, attention, and stamina. This...

Blog
January 9, 2017
What is a medical device? A medical device is any product that is intended to be used alone or in combination with another product, in the diagnosis of diseases or in the...

Blog
December 29, 2016

The national e-Governance plan (NeGP) was launched in 2006 as an initiative that the Government of India has implemented to make all the government services accessible online. It has been activated in...

Blog
November 29, 2016

The Central Government of India defines a Medical Device as a medical instrument for external or internal use in the prevention, diagnosis or treatment of a condition or disease. It also includes...

Blog
November 24, 2016
BIOLOGICS AND BIOSIMILARS Biologics are medicinal products which are mainly composed of living tissues or cells. Sometimes, they are living entity- derived products used for the treatment or prevention of a disease....

Blog
November 10, 2016

There is a sudden rise of e-commerce food business operators (FBOs) in India due to digitalization. E-commerce FBOs includes any kind of sellers, vendors, importers, manufacturers, processors, packagers, restaurants, hotels who market...

Blog
October 10, 2016
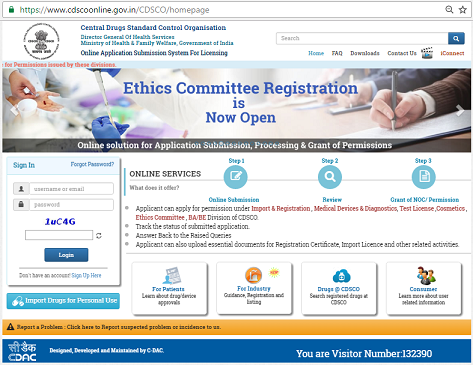
With rapidly growing digitalization in the world of healthcare and its accessory outfits, the Indian Government has chosen to join the foray and ride the digital wave. As part of implementation of...

Blog
September 26, 2016

Food Safety and Standards Authority of India (FSSAI) is actively working in rendering safety and nutritiously balanced food to the people of the country. Recently, the new guidelines for proprietary food in...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding