Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
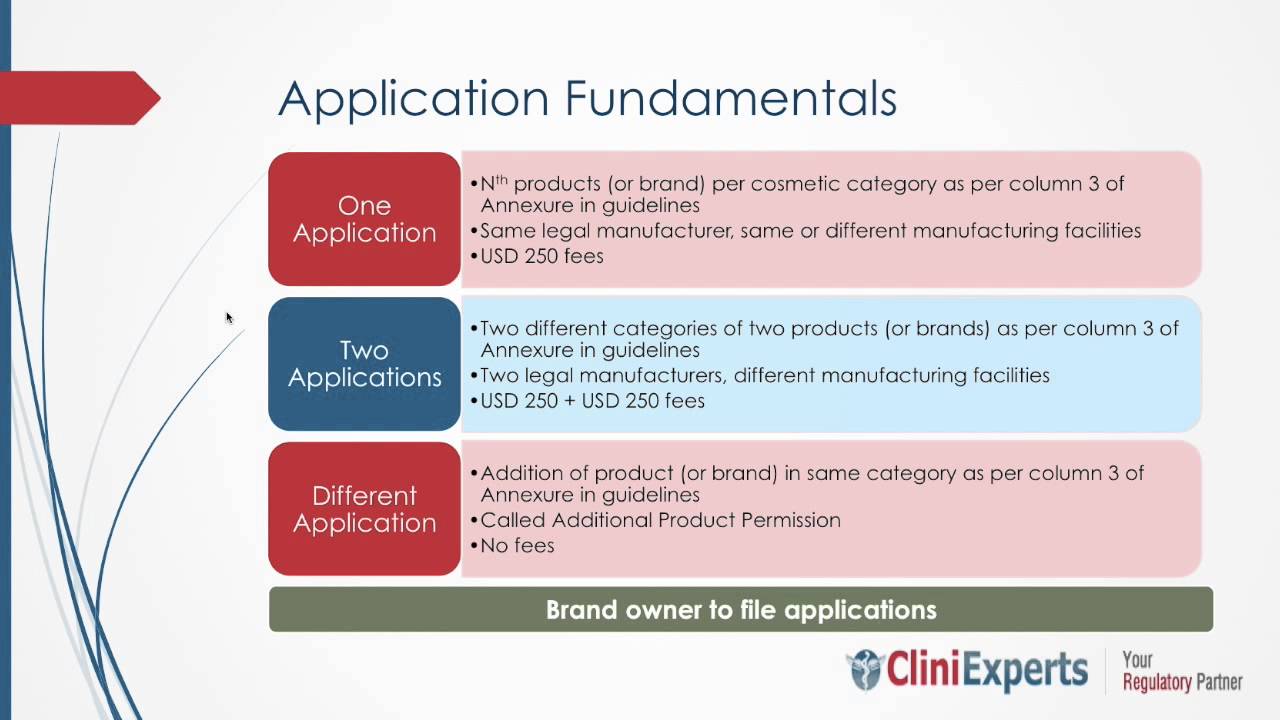
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
September 7, 2016

India has been an attractive destination for clinical trials because of its large population and varied demographics. Despite having tremendous potential, only 1–2% of global clinical trials are being conducted in India....

Blog
September 7, 2016

National List of Essential Medicines The National List of Essential Medicines (NLEM), 2011, has been reviewed and revised by the Core-Committee constituted by the Ministry of Health & Family Welfare (MOHFW),...

Blog
September 7, 2016

As per the Director General of Foreign Trade (DGFT) via. the notification on August 4, 2016, (Notification No. 19 /2015-2020), an amendment in the policy of the import and export of...

Blog
August 30, 2016
The Central Drugs Standard Control Organization (CDSCO) plays an important role in safeguarding and enhancing public health by ensuring the quality, safety, and efficacy of drugs, cosmetics, and medical devices. It is...

Blog
August 3, 2016
stethoscope in two years, preferring to examine his patients with a handheld ultrasound unit to view a patient’s heart in real-time. In 2012 alone, physicians in the U.S. are expected to increase...

Blog
July 29, 2016
In order to strengthen the scientific review and approval of new drugs/devices, the ministry has appointed 12 New Drug Advisory Committee’s (NDAC), Subject Expert Committees (SEC) and 7 Medical Device Advisory Committee’s...

Blog
July 29, 2016
It is acknowledged within the pharmaceutical industry that clinical trials represent the single most expensive aspect of a drug, or treatment, development process. Specifically, clinical trials are enormously resource-intensive and involve repetitive,...

Blog
July 29, 2016
What is a New Drug as per Drug and Cosmetic Rules (D&C)? As per Rule 122 E of the Drug and Cosmetic Rules 1945, a New Drug can be – [1] A...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding