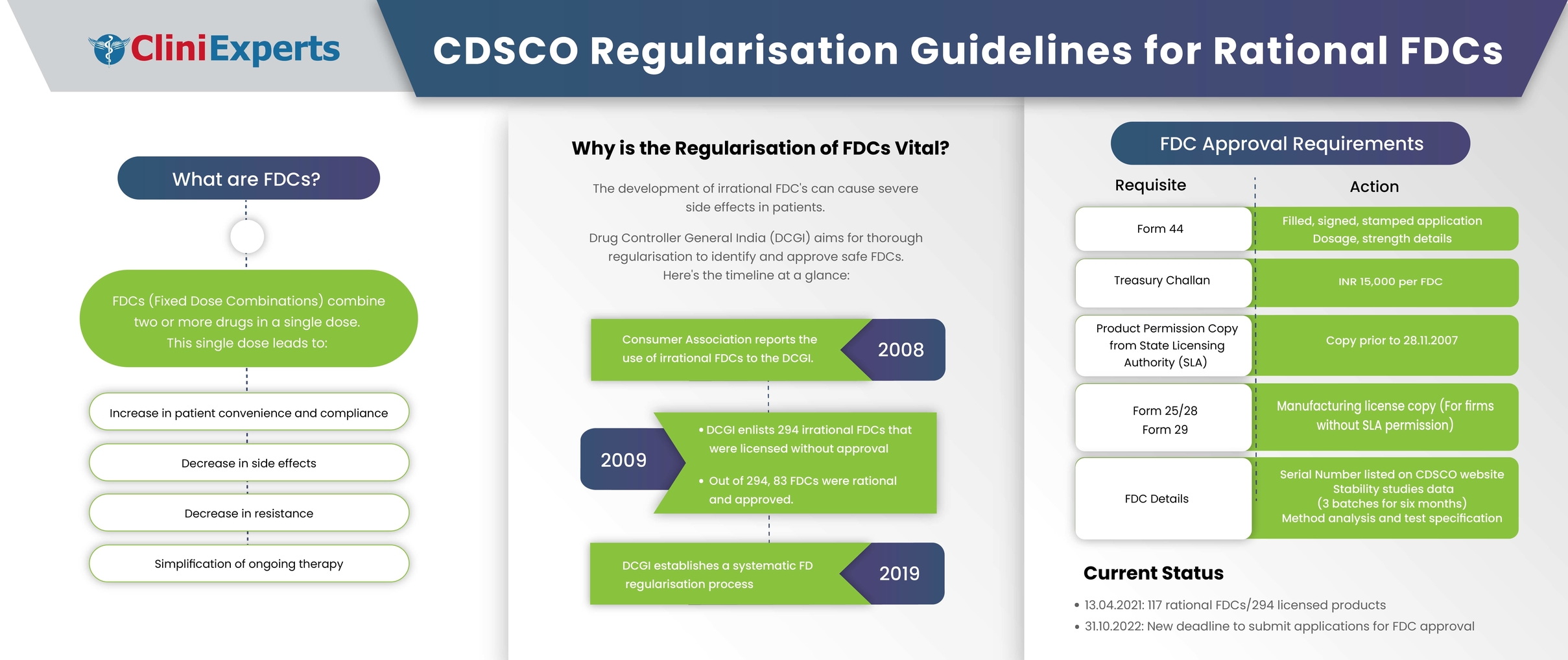
CDSCO GUIDELINES FOR REGULARISATION OF RATIONAL FIXED DOSE COMBINATIONS
Rational fixed dose combinations (FDCs), also known as combination products, are the integration of two or more drugs in a single dosage form. The Food and Drug Administration (FDA), USA defines a combination product as 'a product composed of any combination or integration of a drug and a device or a biological product, or a device.
Read MoreUnder The Provisions Of The Medical Devices Rule 2017, The DCGI Classifies Medical Devices Pertaining To Rehabilitation
The Drug Controller General India (DCGI) has categorized medical devices pertaining to rehabilitation based on their intended use, associated risks, and other factors provided in the Medical Devices Rules, 2017's First Schedule.
Read MoreBenefits Of Wholesale Drug License In Form 20B & 21B?
A wholesale drug license in Form 20B / 21B has numerous benefits for wholesalers despite the strict rules and regulations of CDSCO and SLA. Since wholesale drug license has a Indian reorganization, so it opens up endless possibilities for businesses.
Read More
All About Medical Device Authorized Agent / Representative
To become an Authorized Agent of Medical Devices in India, a licensed wholesaler must be approved for the sale and distribution of medical devices and should apply for a Medical Device Import License from the Central Licensing Authority.
Read More
DCGI Enlists New Regulation For The Classification Of Medical Devices Pertaining To Cardiovascular
The Drugs Controller General (India) (DCGI), Directorate General of Health Services, has classified medical devices pertaining to cardiovascular based on their intended use, associated risk and other parameters.
Read More
Why Do You Need In-Vitro Diagnostic Kits (IVD) Import License?
In India, the CDSCO reviews IVD kits/reagents for importation. The import license of IVD kits ensures the safety and efficacy of patients and prevent the risks associated with the design, manufacture, and packaging of medical devices. The import license for notified and non-notified In-Vitro Diagnostics kits is obtained in Form MD 15.
Read MoreRegulation/Guidelines
What Are The Cosmetic Regulations In India For Cosmetic License Approval?
According to the Drugs and Cosmetics Act, 1940, and New Cosmetics Rules 2020 and the Rules enacted thereunder, the State Drug Control Department inspects and grant cosmetic license approval for importers and manufacturers in India. Additionally, the import of cosmetics is governed by a registration system administered by the Central […]
Regulation/Guidelines
What are CDSCO Form MD 26 and 27 and the benefits of having the import license for new medical devices?
An import license is a prerequisite for starting a business to sell or distribute medical devices. Having an import license gives a clear idea about the specification and standards of imported Medical devices.
Regulation/Guidelines
How To Get Wholesale Drug Licenses In India?
A step-by-step process to obtain a wholesale drug license for manufacturers – eligibility criteria, required document, validity and process.
