
Regulatory Update
May 21, 2024

Regulatory Update
July 4, 2022

Blog
February 15, 2022

A wholesale drug license in Form 21B is a prerequisite for starting a business to sell or distribute drugs that include biologicals, in-vitro diagnostic kits, and medical devices. In India, the government...

Blog
January 31, 2022

The medical devices market is growing at a fast pace along with the drug market despite all the challenges created by insufficient quality standards and other inconveniences. In India, medical devices are...

Blog
October 27, 2021

Based on the provisions of the Medical Device Rules (MDR) -2017, the Drug Controller General of India (DCGI) has issued a classification of in-vitro diagnostic (IVD) medical devices used in various clinical...

Blog
June 10, 2021
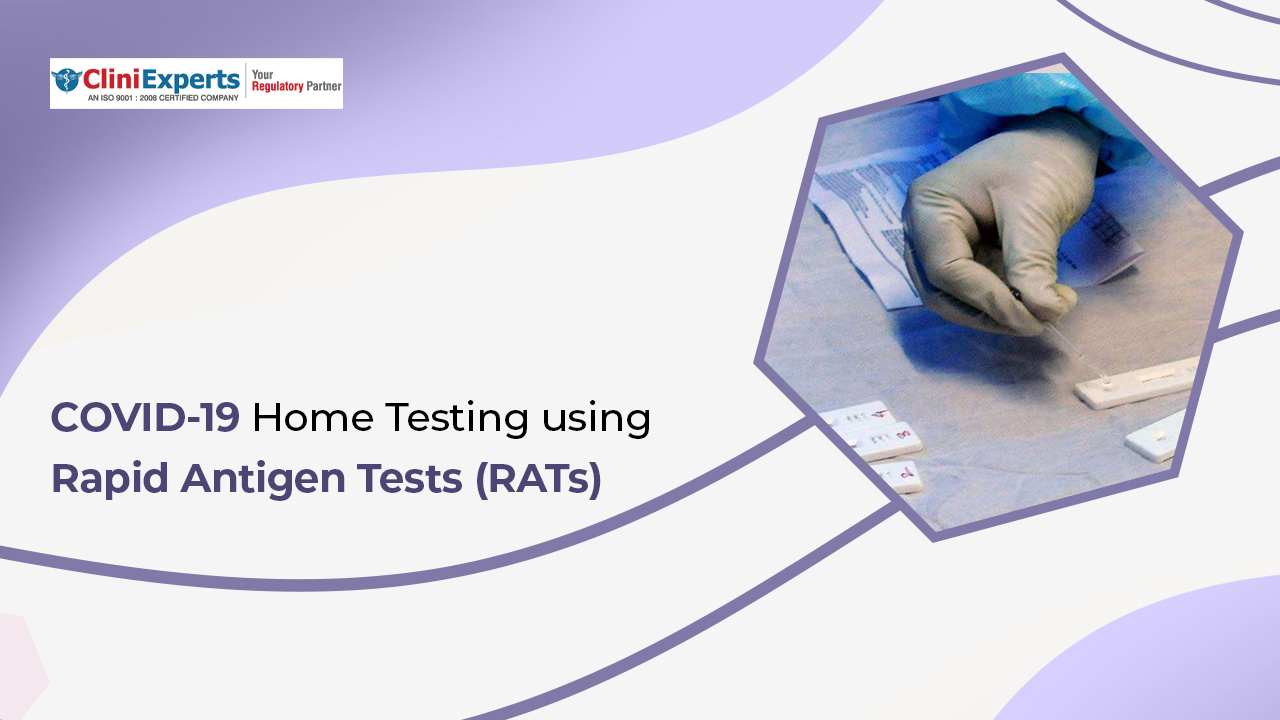
Rapid Antigen tests (RATs) are commonly used to diagnose respiratory pathogens, including influenza virus and SARS-CoV-2 virus. These tests have come to practical use in the diagnosis of the infection in the...

Blog
April 22, 2021

CDSCO has designated the National Institute of Biologicals (NIB), Noida, as Central Medical Device Testing Laboratory as per rule 19 of Medical Device Rules, 2017 released on 1st June 2018.NIB performs tests...

Blog
November 9, 2020

The Central Drugs Standard Control Organisation (CDSCO) has released two notices on September 3, 2020, including the classification of non-notified medical devices and in-vitro diagnostic devices (IVDs). To simplify the classification process...







