
Blog

According to the latest draft gazette notification, G. S. R. 393 (E), released on 25-05-2022, The Ministry of Health and Family Welfare (MoHFW) has circulated a notification by revising Schedule K of...

Blog
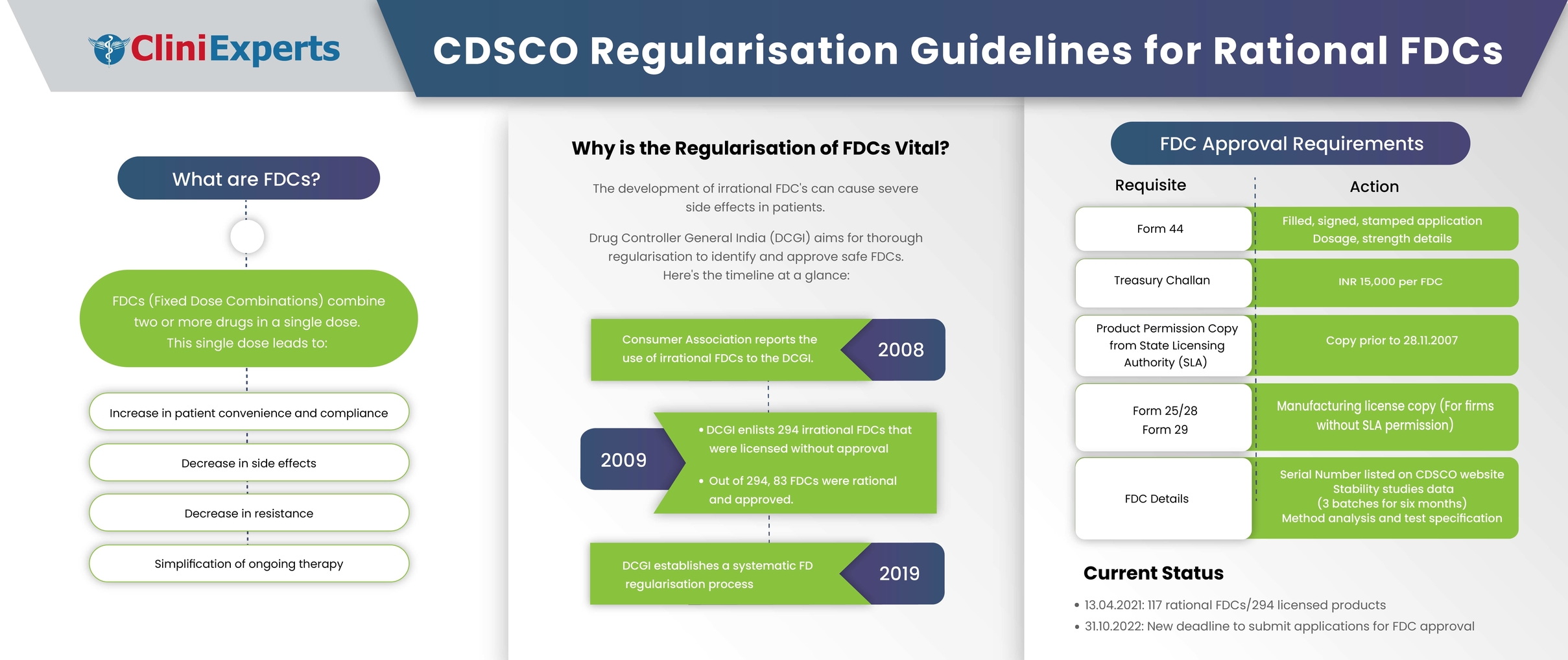
Rational fixed dose combinations (FDCs), also known as combination products, are the integration of two or more drugs in a single dosage form. The Food and Drug Administration (FDA), USA defines a...

Blog

Wholesale Drug License In India - Overview The pharmacy business in India is booming. To ensure consumer safety, the Government keeps changing guidelines that aid streamlining of the processes as well. To...

Blog

A wholesale drug license in Form 21B is a prerequisite for starting a business to sell or distribute drugs that include biologicals, in-vitro diagnostic kits, and medical devices. In India, the government...

Blog

Sanitizers can be of different variants, primarily based on active ingredients, such as soap containing natural fats, detergents, or alcohol-based (Table 1). The Centers for Disease Control and Prevention (CDC) recommends the...

Blog
CDSCO has notified four medical devices, i.e., nebulizer, blood pressure monitoring devices, digital thermometer, and glucometer as drugs. The importers and manufacturers of these 4 medical devices need to take import and...

Blog

Indian pharma market is flooded with irrational Fixed Dose Combination (FDCs) and is an important topic for critical and scientific analysis. The country needs some regulatory laws for FDCs after analysing whether...

Blog

Coronavirus still poses a potential threat to the global population, but no vaccines protect the body against the virus, causing COVID-19. The coronavirus spreads quickly, and majority of the world's population is...