
Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
July 7, 2022

Manufacturing And Product Development Support for Medical Devices / In-Vitro Diagnostic Kit The medical device industry of India is a crucial and integral part of the Indian healthcare industry. It provides preventative,...

Regulatory Update
July 4, 2022

Blog
June 22, 2022

The Ministry of Health and Family Welfare (Department of Health and Family Welfare) under the Government of India, on 18th January 2022, G.S.R.78 (E) released an official gazette with a few new...

Blog
June 20, 2022

Class A Medical Devices / IVDs – Class A medical devices / IVDs are medical equipment that has low risk to the patients and public health risks. Class A medical devices /...

Blog
June 16, 2022
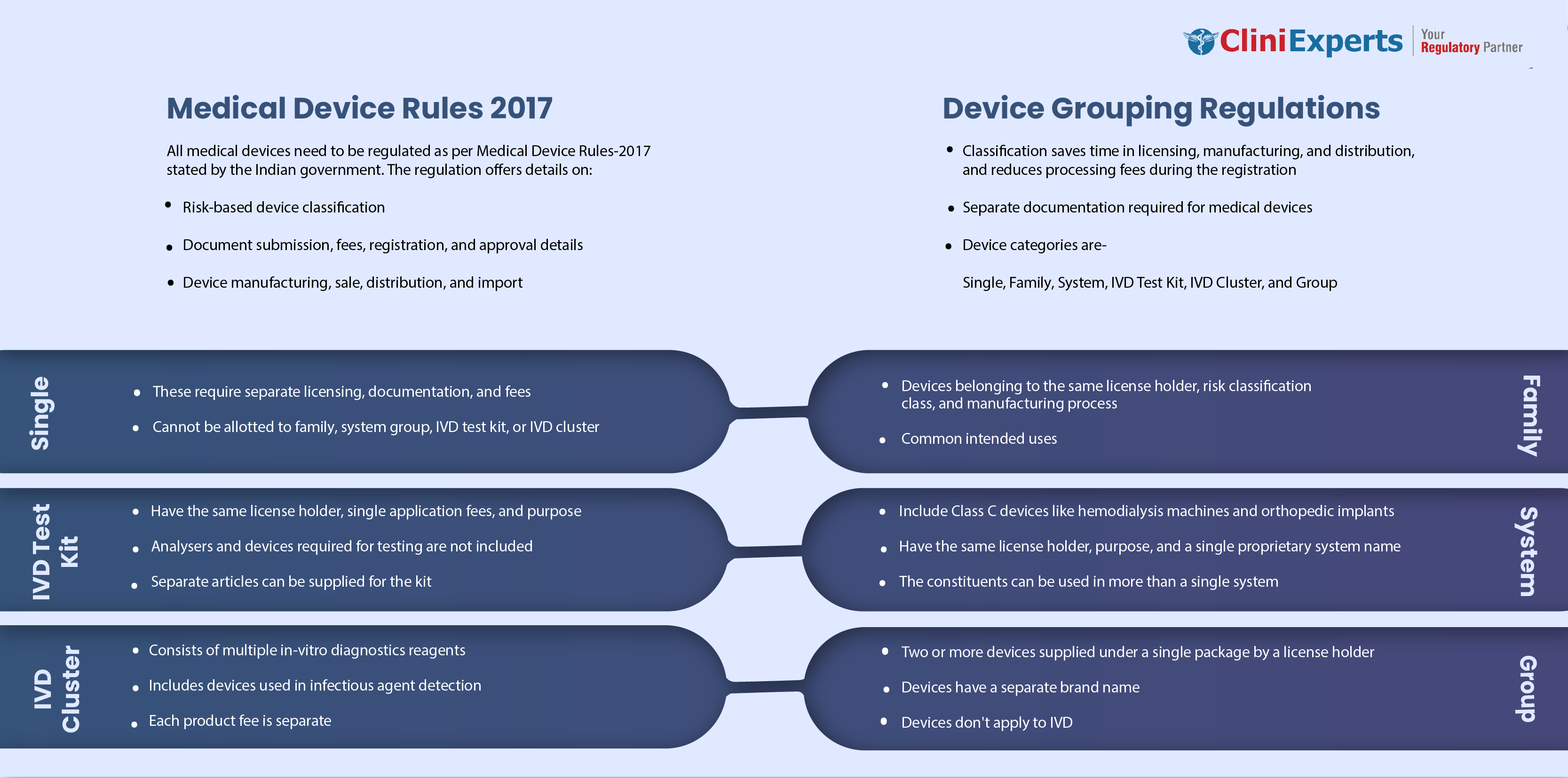
In the year 2017, the government of India announced that all the medical devices in India would be regulated as per Medical Device Rules-2017 (MDR-17), which gives a clear idea about the...

Blog
April 14, 2022

What Is A Medical Device Authorized Agent / Representative? To register/sell a medical device in India, a Foreign Manufacturer must grant a Power of Attorney to a person/company in India who is...

Blog
March 22, 2022

An import license for new medical devices (Form MD 26 & MD 27) is a prerequisite for starting a business to sell or distribute medical devices. Having an import license gives a...

Regulatory Update
February 28, 2022







