Complete Procedure to Meet the CDSCO License Mandate of Class C & D Non-Notified Medical Devices
Medical Device
October 25, 2023
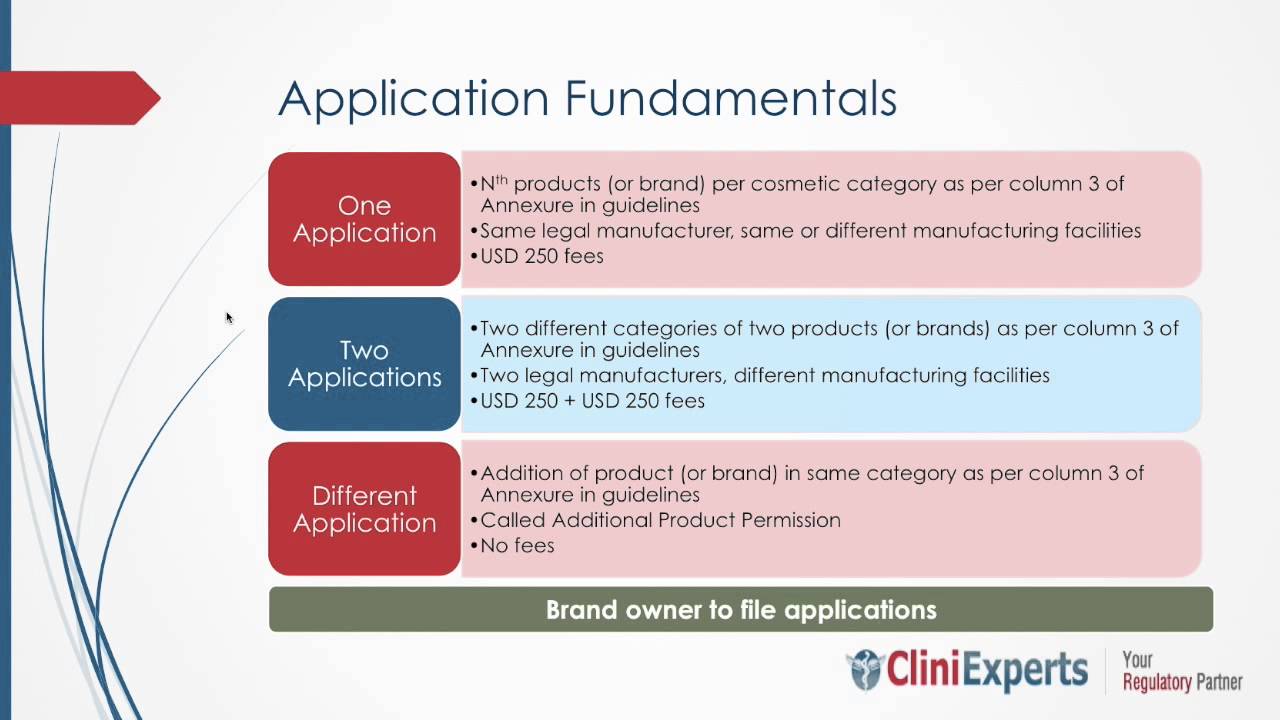
Overview of the CDSCO License Mandate The Central Drugs Standard Control Organisation (CDSCO) is the regulatory body that manages medical device registration in India under the Ministry of Health & Family Welfare. The main aim of CDSCO is to ensure that medical devices are...
Read More


Blog
November 7, 2019

The Central Authority, Central Drugs Standard Control Organisation (CDSCO) came out with Frequently Asked Questions (FAQs) on the Import and Registration of Drugs in India. Let’s take a look at the...

Blog
July 30, 2019

Internet has led to an exponential growth in awareness. You are more tuned to “Googling” for information and clearing your doubts. There is a growing tribe of consumers who are alert,...

Blog
July 23, 2019

Imagine this scenario You have researched the market and identified certain needs You have mapped it to your core competency, strengths, resources You have invested money and taken risk You have identified...

Blog
July 19, 2019

A report by TechSci Research states that the Indian cosmetics market is expected to grow at a CAGR of 6% in the period from 2019 to 2024. There is growing demand for...

Blog
July 15, 2019

India is an emerging economy with high GDP growth rate and extremely lucrative market for consumer goods manufacturers like cosmetics. A report by TechSci Research states that the Indian cosmetics market is...

Blog
July 11, 2019

Cosmetic items fall under the purview of the Ministry of Health and Family Welfare in India. The overarching Drug and Cosmetics Act 1940 and the Rules 1945 (last amended on 01.12.2016) regulate...

Blog
July 8, 2019

Have you ever thought that – The ubiquitous lipstick often reaches our alimentary canal as we eat and drink. The eye-liner permeates into the sensitive areas of our eyes. The gases and...

Blog
May 30, 2019
Ministry of Health and Family Welfare of India notified the publication of rules for New Drugs and Clinical Trials, 2019 with an aim to ease and boost clinical research and trials in...
End-to-End Regulatory Solutions for Domestic and International Markets
ENQUIRE NOW
KNOW MORE, LEARN MORE, ENGAGE MORE.
“Video-Only” Resource For Ease Of Understanding