
ISO 13485 Medical Devices Certification: Medical Device ISO Standards
Medical Device ISO 13485 Certification is a systematic framework for manufacturers, importers and distributors that maintains the quality and standard of regulatory compliance for medical devices. It indicates that a company has implemented a Quality Management System (QMS).
Read MoreHealth Ministry Draft Notification To Add 16 Drugs as OTC Under Schedule K of Drugs Rules, 1945
Ministry of Health and Family Welfare released a draft notification to add 16 drugs as OTC under schedule k of drugs rules, 1945.
Read MoreNew Rules added by the CLA on suspension and cancellation of license of imported Medical Devices.
The Ministry of Health and Family Welfare (Department of Health and Family Welfare) under the Government of India, on 18th January 2022, G.S.R.78 (E) released an official gazette with a few new rules on the import of medical devices in India, and the final amendment of this rule G.S.R.174 (E) was released on 4th March 2022.
Read More
Guidelines for Prevention of Consumers from Misleading Advertisements and Endorsements for Misleading Advertisements, 2022
India’s central consumer protection body published rules for preventing misleading commercials and endorsements for misleading advertisements 2022 on June 9th, 2020. CCPA announced the Guidelines to fulfill the duties given to CCPA by Section 18 of the Consumer Protection Act, 2019. Section 2(28) of the 2019 Consumer Protection Act defines […]
Read More
Manufacturing And Product Development Support For Class A Medical Devices/IVD’s
Get manufacturing and product development support for class a medical device/IVD’s by CliniExperts’ professionals including product development, facility association, documentation, quality management, manufacturing license, and audit and inspection support.
Read More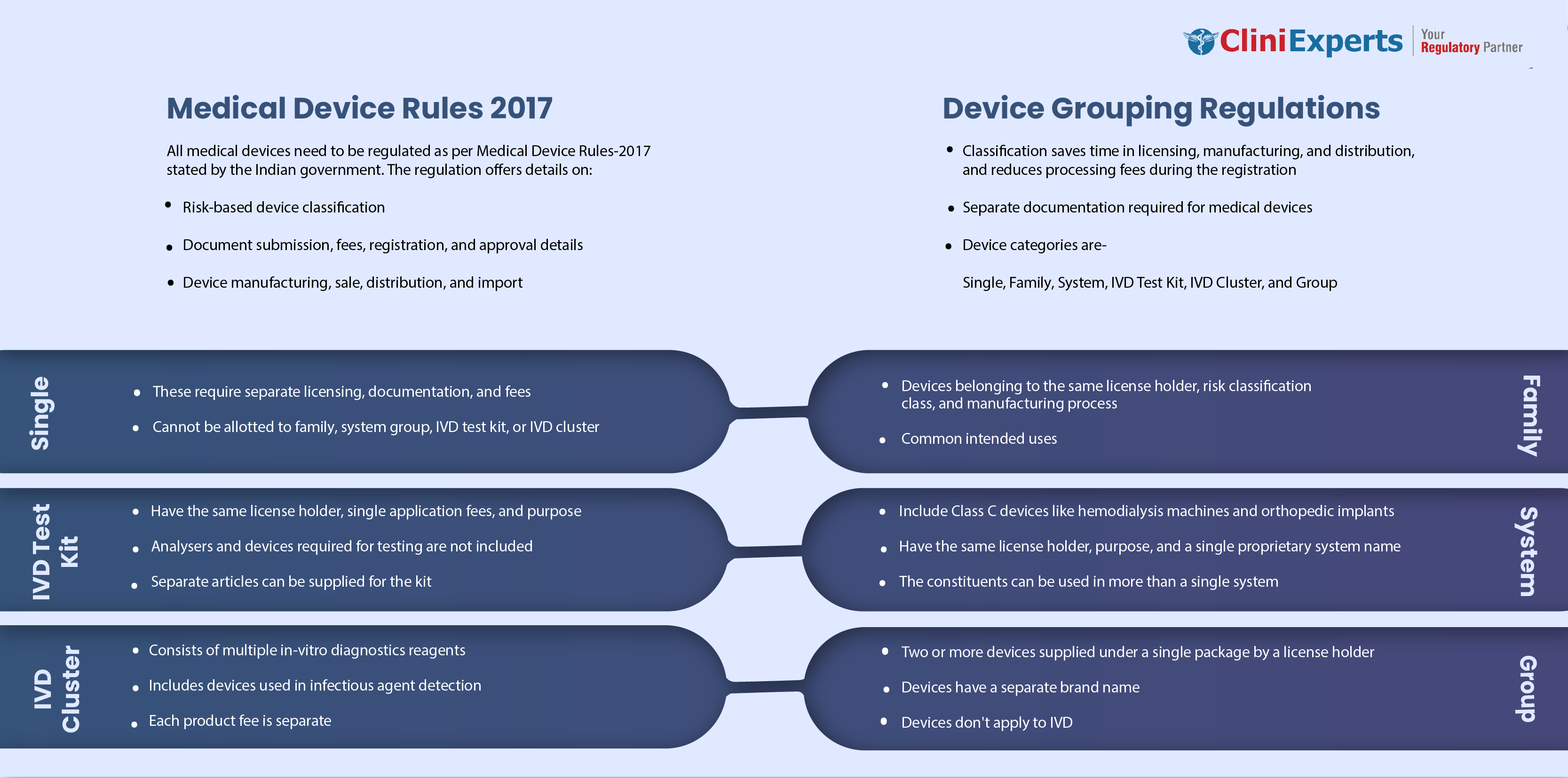
Medical Device Grouping as per MDR 2017
In the year 2017, the government of India announced that all the medical devices in India would be regulated as per Medical Device Rules-2017 (MDR-17), which gives a clear idea about the Classified the medical device based on risks. Procedure related to document submission, registration, fees, and approval of devices. Details related to manufacturing, import, sale, and distribution of medical devices.
Read MoreRegulation/Guidelines
Draft of the new bill on New Drugs, Medical Devices and Cosmetics, 2022
The new draft bill consists of a few new definitions such as clinical trials, over-the-counter drugs, manufacturers, cosmetics, medical devices, new drugs, bioavailability, investigational new drugs, imported spurious drugs, predicate devices and many others. The new draft bill consists of seven chapters and nine schedules. Chapter II of the draft […]
Regulation/Guidelines
CLASS B MEDICAL DEVICE/IVD MANUFACTURING AND PRODUCT DEVELOPMENT SUPPORT
Get class B medical devices manufacturing & product development support by CliniExperts’ professionals including class B product development, documentation, quality management, manufacturing license, audit and inspection support.
Regulation/Guidelines
Manufacturing And Product Development Support for Medical Devices / IVDs (Class C & Class D)
Get Manufacturing And Product Development Support for Medical Devices / IVDs for Class C and Class D by CliniExperts’ professionals including product development, facility designing, documentation, quality management, manufacturing license, and audit and inspection support.
